Anatomy of the Breast
The breast or mammary gland is a modified sweat gland that has the specific function of milk production. An understanding of the basic anatomy, physiology, and histology is important in the interpretation of mammography. With an understanding of the normal breast, one is better able to correlate radiologic-pathologic entities.
Development
The development of the breast begins in the fifth-week embryo with the formation of the primitive milk streak from axilla to groin. The band develops into the mammary ridge in the thoracic area and regresses elsewhere.
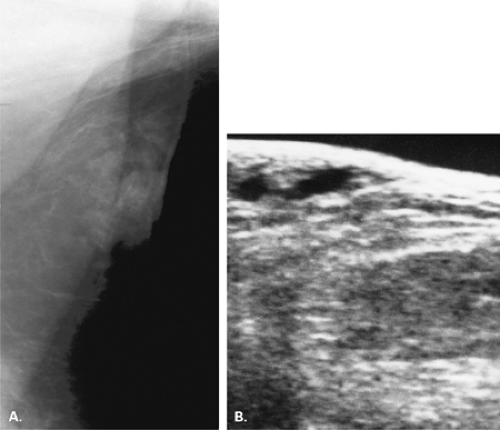
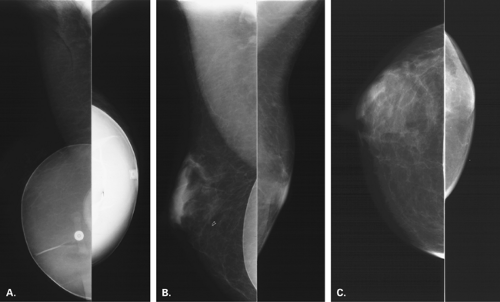
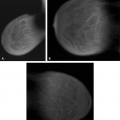
If there is incomplete regression or dispersion of the milk streak, there is accessory mammary tissue present in the adult, which occurs in 2% to 6% of women (1). Accessory breast tissue, particularly in the axillary area, that is separate from the bulk of the parenchyma may be identified on mammography in these women (2) (Fig. 1.1). The orientation of the milk streak is slightly lateral to the nipple above the nipple line and medial to the nipple below the nipple line. Therefore, patients with accessory breasts, accessory parenchyma, or accessory nipples are found to have these in the axillary region or just medial to the nipple in the inferior aspect of the breast or upper abdominal wall (Figs. 1.2, 1.3). In women with accessory breast tissue, changes occur cyclically and with pregnancy and lactation, as they do within the breasts themselves. Therefore, these patients may note that areas of accessory breast tissue may enlarge with pregnancy and may produce milk when the patient is lactating if there is a duct orifice or nipple present.
At 7 to 8 weeks of embryologic development, there is an invagination into the mesenchyma of the chest wall. Mesenchymal cells differentiate into the smooth muscle of the nipple and areola (1,3). At 16 weeks, epithelial buds develop and branch. Between 20 and 32 weeks, placental sex hormones entering the fetal circulation induce canalization of the epithelial buds to form the mammary ducts. At 32 to 40 weeks, differentiation of the parenchyma occurs, with the formation of the lobules (3,4).
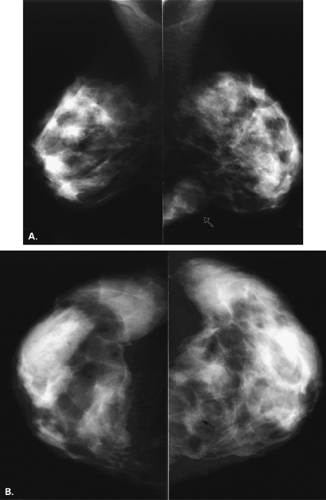
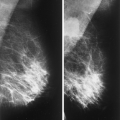
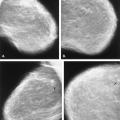
The mammary gland mass increases by fourfold, and the nipple-areolar complex develops (1). Developmental anomalies include polymastia (accessory breasts along the milk streak), polythelia (accessory nipples), hypoplasia of the breast (Fig. 1.4), amastia (absence of the breast), and amazia (absence of breast parenchyma) (Fig. 1.5) (1). Systemic or iatrogenic influences in childhood may be related to breast hypoplasia or amazia. Iatrogenic causes of amazia include excision of the breast bud during biopsy of the prepubertal breast and the use of radiation therapy to the chest wall during childhood (1) (Fig. 1.6).
During puberty in girls, the release of follicle-stimulating hormone and luteinizing hormone by the pituitary causes release of estrogens by the ovary. Hormonal stimulation induces growth and maturation of the breasts. In early adolescence, the estrogen synthesis by the ovary predominates over progesterone synthesis. The physiologic effect of estrogen on the developing breast is to stimulate longitudinal ductal growth and the formation of terminal ductule buds (1). Periductal connective tissue and fat deposition increase (1), accounting for increase in size and density of the breasts.
Structure
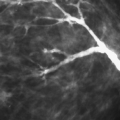
The adult breast is composed of three basic structures: the skin, the subcutaneous fat, and the breast tissue, which includes the parenchyma and the stroma. Beneath the breast is the pectoralis major muscle, which is also imaged during mammography. The breast parenchyma is enveloped by deep and superficial fascial layers; Cooper’s ligaments, the fibrous strands that support the breasts, traverse the parenchyma and attach to the fascial layers. The parenchyma is divided into 15 to 20 segments, with each drained by a lactiferous duct (Fig. 1.7). The lactiferous ducts converge beneath the nipple, with about 5 to 10 major ducts draining into the nipple. Each duct drains a lobe composed of 20 to 40 lobules (Fig. 1.8).
The microanatomy of the breast was described by Parks in 1959 (4). Each lobule is 1 to 2 mm in diameter and contains a complex system of tiny ducts (the ductules), which terminate in blind endings. The ductules can respond to hormonal stimulation of pregnancy by proliferation and formation of alveoli (3). Two types of stroma are present: the perilobular connective tissue, which contains collagen and fat, and the intralobular connective tissue, which does not contain fat (4).
Wellings et al. (5) further classified the microstructure of the normal breast into the terminal duct lobular unit (TDLU) (Fig 1.9). Small branches of the lactiferous ducts lead into terminal ducts that drain a single lobule. The terminal duct is composed of the extralobular segment and the intralobular segment. The lobule is composed of the intralobular terminal duct and the blindly ending ductules (5). The ductules are lined by a single layer of epithelial cells and a flattened peripheral layer of myoepithelial cells (5). A loose fibrous connective tissue stroma supports the ductules of the lobule.
The TDLU is a hormone-sensitive gland varying from 1 to 8 mm in diameter in the nonpregnant state and having the potential of milk production (6). The lobules normally regress at menopause, leaving blunt terminal ducts; however, in women older than 55 years with breast cancer, Jensen et al. (6) found the TDLUs remain well developed.
The work of Wellings et al. (5) has suggested that the TDLU is a basic histopathologic unit of breast from which many benign and malignant lesions arise. Fibroadenomas, sclerosing adenosis, apocrine cysts, lobular hyperplasia, and lobular carcinoma in situ are thought to develop in the lobule itself; ductal hyperplasia and ductal carcinoma in situ develop in the TDLU. Solitary intraductal papillomas, epithelial hyperplasia of the larger ducts, and duct ectasia occur in the main lactiferous ducts (4). Correlative studies between radiographic and histologic appearances of the breast parenchyma suggest that small nodular densities on mammography represent lesions of the terminal duct lobular units and that linear densities are due to periductal and perilobular fibrosis (7).
Blood Supply and Lymphatic Drainage
The primary arterial supply to the breast is from the perforating branches of the internal mammary and lateral
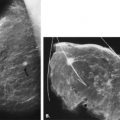
thoracic arteries. Minor contributions to the blood come from the branches of the thoracoacromial, subscapular, and thoracodorsal arteries (1). Venous drainage is primarily via branches of the internal mammary, intercostal, and axillary veins. If there is obstruction of the subclavian vein, collateral drainage produces dilated, tortuous vascular structures, easily visible on mammography (Fig. 1.10).
thoracic arteries. Minor contributions to the blood come from the branches of the thoracoacromial, subscapular, and thoracodorsal arteries (1). Venous drainage is primarily via branches of the internal mammary, intercostal, and axillary veins. If there is obstruction of the subclavian vein, collateral drainage produces dilated, tortuous vascular structures, easily visible on mammography (Fig. 1.10).
Lymphatic drainage is via the superficial plexus to the deep plexus to the axillary and internal mammary lymph nodes. The low axillary nodes are often visible on mammography, as are small intramammary nodes. It is unusual to identify on mammography intramammary nodes in a location other than the superficial region of the middle- to upper-outer quadrant of the breast.
Musculature
The breast lays over the musculature of the chest wall: the pectoralis major and minor muscles. The pectoralis major muscle has its origins at the anterior medial surface of the clavicle, the sternum, and the aponeurosis of the external oblique muscle and its insertion on the proximal humerus. The pectoralis major muscle, therefore, lies obliquely over the chest wall. This angle of obliquity varies with the body type of the individual. A parameter for a well-positioned mediolateral oblique (MLO) mammographic view is that the pectoralis major muscle is visible from the axilla down to the level of the nipple. Before positioning the patient for the MLO view, the




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree