Anal Cancer
Anal cancer is responsible for approximately 2.1% of digestive system malignancies with 6230 new cases estimated in the United States in 2012 (1). Anal cancer was once believed to be caused by chronic inflammation of the anal canal and treated with abdominoperineal resection (APR). Research has now shown that the development of anal cancer is associated with human papillomavirus (HPV) infection and has a pathophysiology similar to that of cervical cancer. Concurrent chemotherapy and external beam radiation therapy (EBRT) regimens have essentially replaced APR as primary treatment and have allowed for a great majority of patients to be cured with preservation of the anal sphincter.
ANATOMY AND HISTOLOGY
The anal canal extends from the junction of the puborectalis portion of the levator ani muscle and the external anal sphincter to the anal verge. The length of the canal averages 4 cm. The anal canal is divided by the transitional zone, or dentate line, which represents the transition from squamous mucosa to glandular mucosa. There is no easily identifiable landmark between the rectum and anus, so clinicians should rely on the pathologic classification of tumors in this area rather than surgical or endoscopic classification. Anal cancers are primarily keratinizing or non-keratinizing squamous cell carcinomas. Adenocarcinomas of the anal canal comprise about 20% of anal tumors, and share the natural history of rectal adenocarcinomas and should be treated as such.
There are two sites of lymphatic drainage from the anal canal. Tumors above the dentate line drain to the perirectal and perivertebral nodes, while tumors below the dentate line drain to the inguinal and femoral lymph nodes. For this reason, patients who present with anal masses should undergo examination of the inguinal lymph nodes, and patients who present with squamous cell cancers in the inguinal lymph nodes should be evaluated for primary anal tumors.
EPIDEMIOLOGY
The incidence of anal cancer has been rising in the United States. In a review of the SEER database from 1973 to 2000, the incidence of anal cancer in men and women has risen from 1.06 and 1.39 per 100,000 persons to 2.04 and 2.06 per 100,000 persons (2). In 2013, an estimated 7,060 cases were diagnosed in the United States. Although the incidence of anal cancer has risen in both sexes, the rate of rise for men is higher, particularly in patients diagnosed with HIV (3, 4). This increase in incidence may be due to better screening techniques and an increased rate of risk factors for anal cancer within the U.S. population that has occurred over time. Survival for patients with anal cancer is consistently worse for men compared to women and for black patients compared to white patients. Several risk factors have been associated with anal cancer:
• HPV infection
• History of genital warts
• Lifetime number of sexual partners
• Receptive anal intercourse
• History of cervical dysplasia or cancer
• History of previous sexually transmitted disease
• Human immunodeficiency virus (HIV) infection
• Cigarette smoking
• Chronic immunosuppression
Anal cancer risk factors mirror risk factors of sexually transmitted diseases, and are due to the link between HPV and anal cancer. Similar to cervical dysplasia and cancer, HPV can cause premalignant anal squamous intraepithelial lesions (ASIL), which can be low grade (LSIL) or high grade (HSIL). Progression of ASIL to invasive anal cancer is influenced by HIV seropositivity, low CD4 count, infection with multiple HPV serotypes, serotype of HPV infection, and high levels of DNA of high-risk serotypes. As with cervical cancer, HPV type 16 is the most frequently isolated serotype in HSIL and invasive anal cancer, present in 30%–75% of cases, and HPV types 6, 11, and 18 are present in 10% of cases.
Although not completely clear, there does seem to be a relationship between HIV infection and anal cancer. Multiple studies have suggested that anal cancer is increasingly prevalent in people with HIV infection (3, 4). Studies have noted an increase in the incidence of HPV infection, ASIL, HSIL, and anal cancer in HIV positive patients compared to HIV negative patients. However, it is difficult to control for separate risk factors including receptive anal intercourse and prior HPV infection in these studies. Unlike traditional AIDS-related malignancies, risk of anal cancer in HIV positive patients does not seem to correlate with worsening immunosuppression. In addition, the incidence of anal cancer has continued to increase in the age of widespread use of highly active antiretroviral therapy (HAART) (4), while the incidence of AIDS-related malignancies such as Kaposi’s sarcoma and non-Hodgkin lymphoma has decreased. A possible explanation is that HAART allows for longer survival with HIV, but does not control HPV infection, allowing more time for HPV infection to create progressive dysplasia.
Individuals with non-HIV causes of chronic immunosuppression, such as renal transplant patients and patients on chronic glucocorticoid therapy, appear to be at an increased risk for ASIL and anal cancer, typically associated with persistent HPV infection. Several case-controlled studies have also noted an increased risk of anal cancer in smokers, particularly current smokers. Cigarette smoking is thought to act as a co-carcinogen.
SCREENING
Given the known high-risk groups for anal cancer, several studies have addressed screening in these populations. Similar to the cervical Papanicolaou smear, anal swabs for cytology are a possible screening method for ASIL and anal cancer. Sensitivity of anal cytology is in the range of 50%–80%, with sensitivity being higher in the HIV positive population. Studies of the potential cost-effectiveness of screening have found that screening HIV positive and HIV negative homosexual and bisexual men every 2–3 years would be cost effective and have life expectancy benefits (5, 6). Other groups where possible benefit of screening has been suggested include all HIV positive individuals, women with a history of cervical dysplasia or cancer, and transplant recipients.
With the development of HPV vaccines, there has been burgeoning interest in the use of the quadrivalent HPV vaccine in the prevention of anal cancer, particularly among high-risk patients. In one study of 602 healthy men who have sex with men (MSM) in the 16–26 year age group, men randomized to receive the quadrivalent HPV vaccine had a lower rate of anal intraepithelial neoplasia (13 per 100 person-years) compared with the placebo group (17.5 per 100 person-years). The rate of grade 2 or 3 AIN associated with oncogenic HPV infection was reduced by 54% in the vaccinated group (7). The vaccine was 94% efficacious against persistent anal HPV-16, and 100% against persistent anal HPV-18 infection. The vaccine was also found to decrease the 2-year recurrence rate of precancerous high-grade anal intraepithelial neoplasia (8). Longer follow-up will be necessary to determine whether this will translate into decreased incidence of anal cancer.
DIAGNOSIS
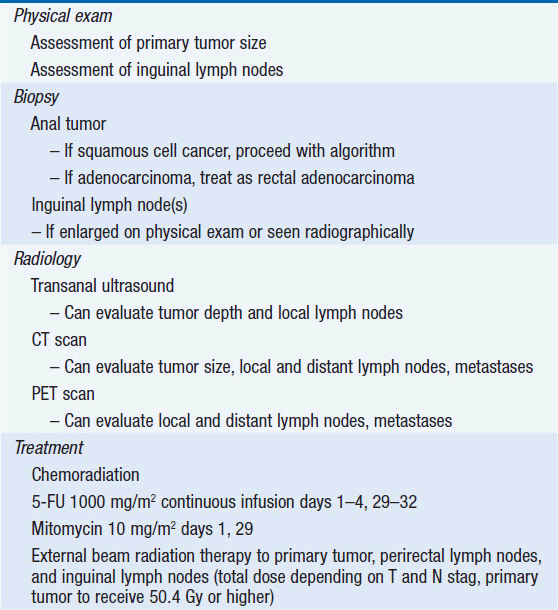
Diagnosis of anal cancer is based on clinical symptoms, physical exam, and biopsy. Patients can present with symptoms of pain, itching, bleeding, discharge, or anal irritation. Patients may also have tenesmus or, with larger tumors, obstructive-type symptoms. Physical exam should include a rectal exam to fully assess the size and location of the tumor and inguinal lymph node exam. Biopsy confirmation to assess histology of the anal mass as well as fine needle aspiration of enlarged inguinal lymph nodes should also be performed. Transanal ultrasonography may be used to evaluate depth of tumor invasion. CT scanning of the abdomen and pelvis can also be used to assess tumor size, invasion, lymph node involvement, and metastatic disease. PET scans or integrated PET/CT scans have also been shown to improve the sensitivity of detecting inguinal lymph node and distant metastases, which may impact treatment recommendations and radiation treatment fields (9, 10).
STAGING
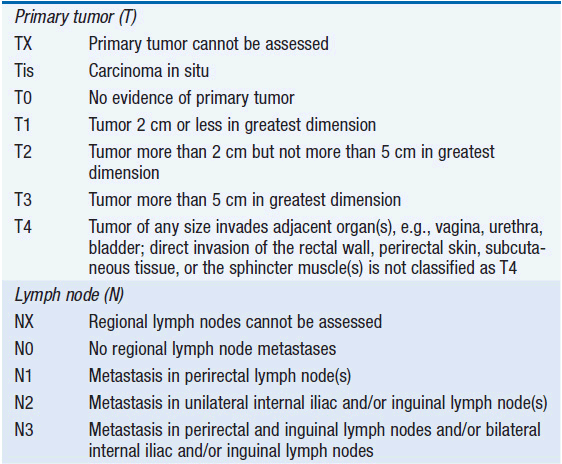
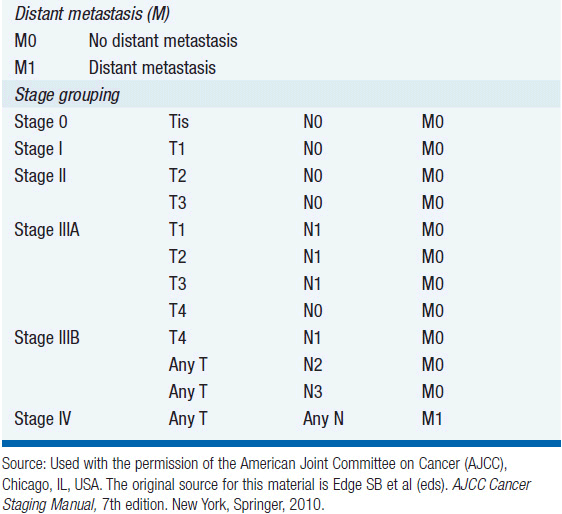
The American Joint Committee on Cancer (AJCC) and the International Union Against Cancer have established a tumor-node-metastasis (TNM) staging system for anal cancer (Table 49-1). Since the primary treatment modality for anal cancer is nonsurgical, staging is based on physical exam, fine needle aspiration of suspicious lymph nodes, and radiologic data. For this reason, the AJCC staging system is based on tumor size rather than depth of invasion. Patients with T1 or T2 lesions have an 80%–90% 5-year survival rate, whereas patients with T4 lesions have less than a 50% 5-year survival rate. For patients with lymph node metastases, the 5-year survival rate is significantly worse at 25%–40%. At presentation, 50%–60% of patients have a T1 or T2 lesion, and 12%–20% are node-positive. The probability of nodal spread is directly related to tumor size and location.
TABLE 49-1 ANAL CARCINOMA STAGING SYSTEM OF THE AMERICAN JOINT COMMITTEE ON CANCER 7TH EDITION (2009)
TREATMENT
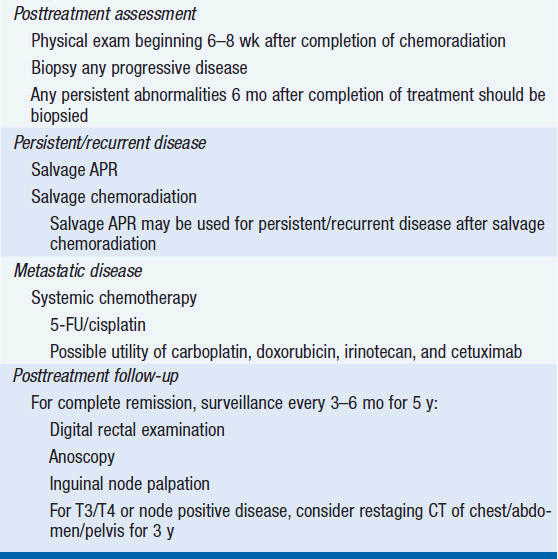
Before 1980, APR, a surgery removing the anorectum and creation of a permanent colostomy, was the treatment of choice for tumors of the anal canal. Surgical series prior to 1980 found the overall 5-year survival rate after an APR to range between 40% and 70%. Patients with large tumors and nodal metastases had poorer outcomes. In an attempt to improve surgical outcome, Nigro and colleagues at Wayne State evaluated preoperative chemotherapy with 5-fluorouracil (5-FU) 1000 mg/m2 continuous infusion days 1–4 and 29–32 and mitomycin 10–15 mg/m2 day 1 combined with EBRT to 30 Gy (11). Unexpectedly, the investigators found that the first three patients who received treatment achieved complete responses. Multiple confirmatory studies have found that combined chemoradiation therapy results in a 70%–86% 5-year colostomy-free survival rate and a 72%–89% 5-year overall survival rate. (See Table 49-2 for suggested diagnosis and treatment options.)
 CHEMORADIATION VERSUS RADIATION THERAPY ALONE
CHEMORADIATION VERSUS RADIATION THERAPY ALONE
Two phase III studies have evaluated the relative benefit of chemoradiation compared to radiation therapy alone.
• UKCCCR
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree