INTRODUCTION
Haploidentical stem cell transplantation (haploSCT) from a first-degree-related haplotype-mismatched donor (siblings, children, parents) could expand allogeneic stem cell transplantation (SCT) to a large proportion of patients with hematologic malignancies without an HLA-matched donor (1). As the average family size continues to shrink, the likelihood of finding an HLA-matched related sibling donor continues to decrease (2). Moreover, as the population continues to age, finding a young, healthy sibling donor becomes increasingly less likely. The use of matched unrelated donors (MUDs) is limited by the long time to SCT (median 3-4 months), which makes it difficult to treat patients with more advanced disease in rapid need of SCT. The ethnicity/race of the recipient can also limit MUD transplantation as approximately 30% of Caucasians, 70% of Hispanics, and 90% of African Americans do not have a MUD in the worldwide registries (3).
In contrast to unrelated donor stem cells, haploidentical (or “half-matched”) donors can be available immediately, and there are no costs associated with an unrelated donor search, maintaining a registry, or coordinating logistics with distant donor centers. This is an especially valuable option for the non-Caucasian and mixed-race individuals (3). This approach might also be particularly useful in developing countries that may not have the resources to procure unrelated donor transplants or maintain complex unrelated donor registries. Moreover, haploidentical donors offer the possibility to easily collect donor cells for cellular therapy posttransplant. Over the recent decade, significant breakthrough advances in controlling alloreactivity have been made and important steps taken toward graft engineering and posttransplant cellular therapy, approaches that changed dramatically the landscape of haploSCT. Improved haploidentical transplant outcomes represent a major advance in SCT that has practically eliminated the limitation of donor availability for allogeneic SCT.
COMPLETE T-CELL DEPLETION: CONTROL OF GRAFT-VERSUS-HOST DISEASE WITH A HIGH TREATMENT-RELATED MORTALITY
Historically, unmanipulated T-cell-replete haploSCT grafts with conventional graft-versus-host-disease (GVHD) prophylaxis used in the late 1970s were associated with intense bidirectional alloreactivity and unacceptably high morbidity and mortality rates due to hyperacute GVHD and graft rejection (4,5,6). This led in the 1980s to the development of complete ex vivo depletion of T cells using CD34-selected grafts. Complete T-cell depletion has been associated with a lower incidence of acute GVHD (aGVHD); however, this caused delayed immune recovery and was associated with a high nonrelapse mortality (NRM) from infections and higher disease relapse rates given the decreased graft-versus-leukemia effect, as well as a higher rate of graft rejection (7,8,9). While graft rejection was partially overcome with “megadoses” of CD34 cells (typically >107 CD34+ cells/kg) and a myeloablative conditioning regimen (including total-body irradiation [TBI], cyclophosphamide, thiotepa) with severe T-cell depletion of the graft, immune recovery remained delayed, leading to high NRM rates in excess of 40% (10). Improved results with this approach have been reported in some centers with selective depletion of alpha-beta T cells. T-cell depletion strategies have been most successful in children (11). We have used this approach with a different conditioning regimen (fludarabine, melphalan, and thiotepa) and showed that most patients died of NRM related to infectious complications (12).
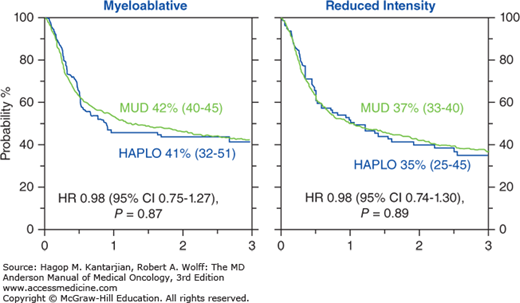
During the last decade, several advances enabled investigators to selectively deplete alloreactive T cells and successfully control GVHD rates while maintaining memory T cells in the graft to accelerate immune recovery and prevent significant infectious complications posttransplant. Table 15-1 summarizes the major contemporaneous approaches to haploSCT. These advances have improved significantly outcomes of patients treated with haploidentical donors, with outcomes now similar to matched transplants (Fig. 15-1) (12a). Here, we summarize the recent developments with this type of transplant, focusing on advances made at the MD Anderson Cancer Center (MDACC).
| Approach | Rationale | Stage of the Clinical Development |
|---|---|---|
| High-dose posttransplantation cyclophosphamide | • Eliminating only the alloreactive T cells • Rapid immune recovery with low infectious complications • Acceptable rates of GVHD • Lower cost | Phase II/III |
| Selective αβ T-cell depletion | • Removing αβ T cells that are most responsive for aGVHD • Remaining γδ T cells thought to have an innate immune-like response capability without inducing GVHD | Phase I/II |
| Photodepletion | • Ex vivo depletion of alloreactive T cells with TH9402 that accumulates in activated T cells | Phase I/II |
| Selective CD45RA+ T-cell depletion | • Elimination of CD45RA+ naïve T cells thought to play a major role in GVHD • Preserves memory T cells that are active against infections | Phase I |
BALANCING GRAFT-VERSUS-HOST DISEASE, IMMUNE RECOVERY, AND THE CONCEPT OF SELECTIVE ALLODEPLETION: POSTTRANSPLANT CYCLOPHOSPHAMIDE
The introduction of high-dose posttransplant cyclophosphamide (HDPTCy) for GVHD prevention represented a major turning point for haploSCT. The concept of inducing immune tolerance with posttransplant cyclophosphamide was introduced by Berenbaum and Brown in 1963, showing that the life of a skin allograft can be prolonged with the use of HDPTCy administered 1 to 3 days after the graft (13). Mayumi et al demonstrated that microchimerism and robust tolerance to minor histocompatibility antigens can be achieved in mice receiving allogeneic splenic cells by intraperitoneal high-dose cyclophosphamide administered on day 2 or 3 posttransplant (14). This concept found its best applicability in allogeneic transplantation, particularly in haploidentical transplantation, where HDPTCy induces bidirectional immune tolerance by selectively eliminating the highly dividing alloreactive donor and recipient T cells generated early posttransplant in the setting of a human leukocyte antigen (HLA) mismatched transplant, with decreased rates of GVHD and graft rejection (15). HDPTCy spares stem cells (due to high levels of aldehyde dehydrogenase present in the cells), which reconstitute the recipient’s hematopoiesis (16), and nondividing T cells, including memory T cells. This results in a more rapid immune recovery compared to T-cell-depleted approaches, leading to lower NRM (lower rates of infections) compared with T-cell-depleted haploSCT, as shown by our group (17).
We have used HDPTCy since 2009, soon after the first human trials showed the safety of this approach (18). Initial studies used a nonmyeloablative conditioning regimen with fludarabine, cyclophosphamide, and 2-Gy TBI, which was associated with a low incidence of grades 2 to 4 aGVHD (35%) and NRM (15%) at 1 year. However, a higher relapse rate was observed (18). We then hypothesized that more intense conditioning is needed and can be tolerated, especially for patients with leukemia, and used our melphalan-based conditioning regimen (with fludarabine 120 mg/m2, melphalan 100 to 140 mg/m2 with thiotepa 5 to 10 mg/kg [subsequently changed to 2-Gy TBI]) previously used in T-cell-depleted haploSCT, which had been effective in inducing remission in most patients with leukemia even with advanced disease (12,17). Updated results for the first 100 patients treated with this regimen showed 3-year PFS rates of 56% to 62% for patients with myeloid malignancies (acute myelocytic leukemia [AML] in complete remission 1/2, myelodysplastic syndrome and chronic myelocytic leukemia in chronic phase) and lymphoma, and 1-year NRM rates of 12% and 22%, respectively (19).
With haploSCT, outcomes improved significantly; we and others have subsequently compared transplant outcomes of patients treated with a haploidentical versus a matched related donor or a MUD (20,21,22). These single-institution studies uniformly showed similar outcomes with haploidentical transplant with HDPTCy and HLA-matched donor SCT (20,21,22). To confirm these findings, we compared outcomes of haploidentical with MUD transplants using the Center for International Blood and Marrow Transplant Research (CIBMTR) database. This retrospective analysis of 2,174 patients with AML showed similar 3-year PFS with haploidentical transplants performed with HDPTCy and MUD transplants (41% vs 42% for myeloablative, P = .87; 35% vs 37% for reduced-intensity conditioning [RIC], P = .89, respectively) (see Fig. 15-1). The incidence of grades 2 to 4 aGVHD was lower for haploidentical compared with MUD transplants (21% vs 42% for myeloablative, 25% vs 35% for RIC) (23). Our group has proposed a large prospective multicenter study comparing transplant outcomes using haploidentical and MUDs to the Bone and Marrow Transplant Clinical Trials Network group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree