Allergic Disorders and Pregnancy
Paul A. Greenberger
The major conditions that the allergist-immunologist diagnoses and treats can occur in the context of gestation or in anticipation of pregnancy. Examples include asthma, allergic and nonallergic rhinitis, acute or chronic rhinosinusitis, nasal polyposis, urticaria, angioedema, anaphylaxis, and immunodeficiency. Goals of managing gravidas should include effective control of the underlying allergic-immunologic conditions, avoidance measures, guidance on medications, action plans or preparedness for emergencies such as acute severe asthma or anaphylaxis, and communication between the physician managing the allergic-immunologic conditions and the physician managing the pregnancy.
Asthma
Asthma occurs in 3.7% to 8.4% of pregnancies in the United States (1–3) and in up to 12.4% of pregnancies in Australia ( 4). Asthma may have its onset during gestation and present as acute severe asthma, requiring hospitalization. Wheezing dyspnea may result in interrupted sleep, persistent coughing, hypoxemia, and even rib fractures during gestation. The sequelae of ineffectively controlled asthma on the gravida can be devastating in that maternal deaths may occur in the most extreme cases ( 5, 6). Other untoward outcomes of asthma during gestation include fetal loss (abortions or stillbirths), increased rate of preterm deliveries (<37 weeks’ gestation), intrauterine growth retardation (<2400 g), antepartum and postpartum hemorrhage, gestational hypertension, pre-eclampsia, oligohydramnios, and hyperemesis gravidarum (1,2, 5, 7,8–10, 11–12,13–17). Not all studies report all the listed complications. There is a troubling report of acute exacerbations of asthma during the first trimester being associated with an increased risk of congenital malformations (18). Repeated episodes of acute severe asthma during gestation have resulted in hypoxemic effects on the fetus. There is a report of pregnancy termination because of life-threatening acute severe asthma ( 19). Conversely, with cooperation between the gravida and physician managing the asthma and effective asthma control, there can be successful outcomes for most women (8–10, 11,13–17,20–22). Prevention of acute severe asthma has been associated with pregnancy outcomes approaching that of the general population (9). Use of inhaled cortiocosteroids (1,2,9, 11,14,20,22,23) has been effective as has prednisone in managing even the most severe cases of asthma during gestation. Some studies have reported small (100 g to 200 g) reductions in birth weight in gravidas who had used prednisone. Other studies have found essentially normal outcomes despite administration of prednisone as long as there was avoidance of hospitalizations and emergency care (9, 11).
Exacerbations of asthma during gestation may result in more hospitalizations than in nonpregnant patients with asthma. One mechanistic explanation is that there is reduced respiratory reserve in gravidas with asthma. It is also possible that they may receive less than recommended treatment because they are pregnant. When a
comparison was made of emergency department treatment, 51 gravidas were compared with 500 nonpregnant women with asthma (24). Presentation peak expiratory flow rate (PEFR) was comparable (51% versus 53%) (24). However, corticosteroids were administered to 44% of gravidas compared with 66% of nonpregnant women (24). Hospitalization rates were similar (24% versus 21%). Unexpectedly, on discharge, oral corticosteroids were prescribed for 38% of gravidas and 64% of nonpregnant women (24). At the 2-week follow-up by telephone, asthma symptoms were reported by 35% of gravidas compared with 23% of nonpregnant women (24). Thus, pharmacotherapy was inadequate, in that oral corticosteroids were less likely to be prescribed with continued asthma symptoms at 2 weeks after emergency treatment. The 2008 American College of Obstetrics-Gynecology Practice Bulletin (2) and the National Asthma Education and Prevention Program Expert Panel Report (1,23) advise oral corticosteroids for treatment of acute episodes of asthma as part of a step-wise approach.
comparison was made of emergency department treatment, 51 gravidas were compared with 500 nonpregnant women with asthma (24). Presentation peak expiratory flow rate (PEFR) was comparable (51% versus 53%) (24). However, corticosteroids were administered to 44% of gravidas compared with 66% of nonpregnant women (24). Hospitalization rates were similar (24% versus 21%). Unexpectedly, on discharge, oral corticosteroids were prescribed for 38% of gravidas and 64% of nonpregnant women (24). At the 2-week follow-up by telephone, asthma symptoms were reported by 35% of gravidas compared with 23% of nonpregnant women (24). Thus, pharmacotherapy was inadequate, in that oral corticosteroids were less likely to be prescribed with continued asthma symptoms at 2 weeks after emergency treatment. The 2008 American College of Obstetrics-Gynecology Practice Bulletin (2) and the National Asthma Education and Prevention Program Expert Panel Report (1,23) advise oral corticosteroids for treatment of acute episodes of asthma as part of a step-wise approach.
Physiologic Changes During Gestation
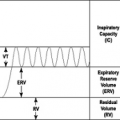
Although the frequency of respiration is not changed, tidal volume increases in pregnancy ( 25, 26). The minute ventilation rises 19% to 50% by late pregnancy ( 25– 27). Vital capacity is unchanged, unless there is an exacerbation of asthma. Oxygen consumption increases by 20% to 32%. Large increases in progesterone and estrogen produce a respiratory alkalosis from greater minute ventilation attributable to increased carotid body sensitivity to hypoxia (28). These changes occur before there is significant enlargement of the uterus. Arterial blood gas concentrations reflect a compensated respiratory alkalosis with pH ranging from 7.40 to 7.47 and partial pressure of carbon dioxide (PCO2) from 25 mm Hg to 32 mm Hg (29, 30). The maternal partial pressure of oxygen (PO2) ranges from 91 to as high as 106 mm Hg ( 30). The near-term alveolar-arterial oxygen gradient is 14 mm Hg in the sitting position compared with 20 mm Hg in the supine position. An explanation for the larger alveolar-arterial oxygen gradient when supine is decreased cardiac output because the enlarging uterus compresses the inferior vena cava which reduces venous return. In the third trimester, gravidas should try to avoid sleeping supine (29).
Total lung capacity is unchanged or reduced by 4% to 6%. The gravida breathes at reduced lung volumes because her residual volume and functional residual capacity are decreased. The diaphragm moves cephalad ( 27). As with the development of maternal hyperventilation, the residual volume and functional residual capacity decline before significant uterine enlargement occurs. The diaphragm flattens during gestation, and there is less negative intrathoracic pressure reported in some studies. One could speculate that early airway closure would occur if there were less negative intrathoracic pressure. Because during episodes of acute asthma, the gravida with asthma generates large negative intrathoracic pressures to apply radial bronchodilating traction, any decline in ability to develop more negative inspiratory pressures would predispose gravidas with asthma to more sudden deterioration because of airway closure.
Bronchial responsiveness to methacholine does not change in a clinically important way; however, a statistically significant change has been reported with PC20 increasing from 0.35 to 0.72 mg/mL from pre-conception to postpartum ( 31). In this study of gravidas with mild asthma, the forced expiratory volume in 1 second (FEV1) improved by 150 mL and the FEV1 increased from 82% to 87% ( 31). The increase in serum progesterone concentration during gestation did not correlate with improvement in bronchial responsiveness ( 32). Although progesterone relaxes smooth muscles of the uterus and gastrointestinal tract, these findings suggest that factors other than progesterone contribute to changes in bronchial responsiveness.
Other Physiologic Changes
Cardiac output increases by 25% at 6 weeks and in later pregnancy can rise 30% to 60% because of the increase in heart rate and reduced vascular resistance ( 30,33,34). The latter results from estrogen supported generation of nitric oxide (35). The decrease in systemic vascular resistance is accompanied by an increase in the heart rate from 10 to 20 beats/minute. Stroke volume increases little; the uterine blood flow rises as much as 10-fold, from 50 mL/min to 500 mL/min at term ( 30). The blood volume increases an average of 1600 mL, and gravidas appear vasodilated as total body water expands by 1 L to 5 L ( 30,33,34, 36). Gravidas are sensitive to overzealous fluid administration. Although correcting any dehydration is indicated, injudicious fluid replacement has resulted in acute pulmonary edema with normal cardiac function. During the latter half of gestation, these changes become manifest because the gravida has increased pre-load (mild volume overload with activation of the renin-angiotensin-aldosterone system), increased chronotropy, and reduced afterload ( 30,33,34).
Fetal Oxygenation
The vascular resistance of uterine vessels (progesterone effect) declines so that there can be the large increase
in uterine blood flow ( 30,34). The fetus survives in a low-oxygen environment with little reserve oxygen store, should the supply of oxygen-rich uterine blood be compromised. Animal and human studies demonstrate reduced fetal oxygenation if there is reduced uterine blood flow that may occur with severe maternal hypotension, hypocarbia, or shock ( 30). Maternal hyperventilation can reduce venous return and shift the maternal oxyhemoglobin dissociation curve to the left. Modest declines in maternal oxygenation seem to be tolerated satisfactorily by the fetus, but substantial degrees of maternal hypoxemia may threaten survival of the fetus. Uterine vessels during gestation are dilated maximally based on experimental data, primarily from pregnant sheep and from some human studies. Uterine vessels do not vasodilate after β-adrenergic agonist stimulation, but do vasoconstrict from α-adrenergic agonists. Some obstetric anesthesiologists administer ephedrine 25 mg to 50 mg intravenously if hypotension occurs during epidural anesthesia. The β-adrenergic effects of ephedrine result in increased cardiac output, which increases systolic blood pressure and maintains uterine perfusion. Intramuscular epinephrine provides primarily β-adrenergic stimulation, whereas intravenous epinephrine results in mostly β and some α effects.
in uterine blood flow ( 30,34). The fetus survives in a low-oxygen environment with little reserve oxygen store, should the supply of oxygen-rich uterine blood be compromised. Animal and human studies demonstrate reduced fetal oxygenation if there is reduced uterine blood flow that may occur with severe maternal hypotension, hypocarbia, or shock ( 30). Maternal hyperventilation can reduce venous return and shift the maternal oxyhemoglobin dissociation curve to the left. Modest declines in maternal oxygenation seem to be tolerated satisfactorily by the fetus, but substantial degrees of maternal hypoxemia may threaten survival of the fetus. Uterine vessels during gestation are dilated maximally based on experimental data, primarily from pregnant sheep and from some human studies. Uterine vessels do not vasodilate after β-adrenergic agonist stimulation, but do vasoconstrict from α-adrenergic agonists. Some obstetric anesthesiologists administer ephedrine 25 mg to 50 mg intravenously if hypotension occurs during epidural anesthesia. The β-adrenergic effects of ephedrine result in increased cardiac output, which increases systolic blood pressure and maintains uterine perfusion. Intramuscular epinephrine provides primarily β-adrenergic stimulation, whereas intravenous epinephrine results in mostly β and some α effects.
The fetal hemoglobin is 16.5 g/L and the oxygen pressure at which hemoglobin is 50% saturated is 22 mm Hg in the fetus, in contrast to 26 mm Hg to 28 mm Hg in the gravida ( 30, 37). Fetal umbilical venous PO2 measurements at term average about 32 mm Hg, with PCO2 49 mm Hg. There is a very large shunt effect of the uteroplacental circulation; this is demonstrated when the gravida inspires 100% oxygen in the absence of acute asthma, fetal umbilical venous PO2 increases to 40 mm Hg and PCO2 is 48 mm Hg ( 37). Such changes in PO2 can be quite important for the fetus in distress, although the uteroplacental shunt is large. For the same incremental increases in arterial PO2, the leftward shift of the fetal hemoglobin oxygen dissociation curve results in larger increases in fetal oxygen saturation than in maternal blood.
In summary, fetal oxygen delivery depends on many factors, but most critical are blood flow (maternal cardiac output) to the uterus, integrity of the placenta, and maternal arterial oxygen content.
Effects of Pregnancy on Asthma
For the individual gravida, it is not always possible to predict the effects of pregnancy on asthma. Studies in the literature report varying degrees of improvement, deterioration, or no change in the clinical course (2,38). Over the past 3 decades, the published reports appear to be rather consistent, with approximately equal proportions of patients being unchanged, improving, or deteriorating. In a review from 1980 of nine studies involving 1,059 pregnancies, 49% of gravidas were unchanged in terms of severity of asthma, 29% improved, and 22% worsened ( 39). A prospective study of 198 pregnancies in 1988 recorded somewhat similar results in that 40% of gravidas had no change in medications, 18% of gravidas required fewer medications, but 42% required more medications ( 40). Similarly, using medication and symptom diary cards, during 366 gestations in 330 gravidas with mild or moderate asthma, asthma was unchanged in 33%, improved in 28%, and worsened in 35% ( 41). In a prospective study of 873 gravidas with asthma from 2003, 44% had no symptoms or treatment during the pregnancy, 32% had intermittent asthma, and 23% were considered to have persistent asthma (mild 13%, moderated 7%, and severe 4%) (13). How effective is the asthma control? In a series of 2,123 gravidas with asthma, about 33% had acute “unscheduled” care ranging from office visits to hospitalizations (42). It is not known if ineffectively controlled asthma contributed, but there is a report of an association between maternal asthma and intellectual disability in children (43). The association also was increased in the presence of maternal diabetes, renal or urinary tract conditions, and epilepsy (43).
Pregnancy in adolescents with asthma has been associated with many emergency department visits and hospitalizations for asthma ( 44). Some adolescents with severe asthma may not benefit from the prescription of anti-inflammatory medications because of poor adherence ( 45). The combination of poverty, inadequate or no prenatal care, limited education, and not being able to make control of asthma a priority can complicate pregnancies at any age of the gravida but especially during adolescent pregnancies.
Cigarette smoking during gestation can have long-term effects. Maternal smoking of 20 or more cigarettes/day in utero was associated with current asthma in 14-year-old girls but not in 14-year-old boys (46). These findings support the persistence of harmful effects of smoking in utero even if the gravida then quits after she delivers. Adverse effects on the child’s lung function, FEV1 and FEF25-75 and FEV1/FVC, have been demonstrated in 7- to 18-year-olds whose mothers smoked during pregnancy or where another member (but not the gravida) smoked during the pregnancy (47). Clearly, gravidas must not smoke during gestation for their own well-being and that of their children.
Choice of Therapy
The approach to therapy includes making an assessment of the level of control, severity, and risks (1,2,23,48,49) (Table 39.1). Specifically, it should be determined (a) whether the gravida has near fatal (potentially fatal) asthma (48,49), (b) whether allergens in the home or workplace are contributing, and
(c) whether the gravida is likely to be adherent to the recommendations provided.
(c) whether the gravida is likely to be adherent to the recommendations provided.
Table 39.1 Goals of Therapy for Management of the Gravida with Asthma | |
|---|---|
|
Avoidance Measures
General avoidance measures include cessation of smoking and preferably recommending that there be no second-hand smoking in the home environment. There should be no or very minimal consumption of alcoholic beverages, cessation of illicit drug use, and avoidance of drugs with teratogenic or harmful potential. Examples of these include tetracyclines (discoloration of infant’s teeth from insufficient production of enamel), sulfonamides in the last trimester (glucose-6 phosphate dehydrogenase G6PD deficiency could cause hemolytic anemia), troleandomycin, clarithromcyin, methotrexate, mycophenolate mofetil, and antibiotics such as quinolones.
When there is allergic asthma, individual avoidance measures should be implemented for animals, dust mites, cockroaches, and fungi. Aspirin and nonsteroidal anti-inflammatory drugs should be withheld in the gravidas with aspirin exacerbated respiratory disease. However, nonselective anti-inflammatory drugs such as ibuprofen or naproxen (50) are considered appropriate for the first 32 weeks of gestation, if indicated for aspirin-tolerant gravidas. Acetaminophen is acceptable.
Medications
There are increasing data to justify the appropriate use of many medications for treatment of asthma and its comorbidities during gestation (Table 39.2) (1,2,13,14,16,21,23




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree