Allergic Bronchopulmonary Aspergillosis
Paul A. Greenberger
Allergic bronchopulmonary aspergillosis (ABPA) is characterized by immunologic reactions to antigens of Aspergillus fumigatus that are present in the bronchial tree and result in pulmonary infiltrates, mucus plugging and proximal bronchiectasis. Allergic bronchopulmonary aspergillosis was first described in England in 1952 in patients with asthma who had recurrent episodes of fever, roentgenographic infiltrates, peripheral blood and sputum eosinophilia, and sputum production containing A. fumigatus hyphae (1). The first adult with ABPA in the United States was described in 1968 (2), and the first childhood case was reported in 1970 (3). Since then, the increasing recognition of ABPA in children (4–10), adults (10–12), corticosteroid-dependent asthmatic patients (13), patients with cystic fibrosis (CF) (11–25), and patients with allergic fungal rhinosinusitis (26–29) is probably the result of the increasing awareness by physicians of this complication of asthma or CF. Diagnosis has been helped by serologic aids such as total serum immunoglobulin E (IgE) (30), serum IgE and IgG antibodies to A. fumigatus (31–33), precipitating antibodies (34), and familiarity with chest radiography and high-resolution computed tomography (CT) findings. Some atypical patients seemingly have no documented history of asthma and present with chest roentgenographic infiltrates, lobar collapse, and peripheral blood eosinophilia (35).
Allergic bronchopulmonary aspergillosis was identified in 6.0% of 531 patients in Chicago with asthma and immediate cutaneous reactivity to an Aspergillus mix (36), whereas 28% of such patients in Cleveland had ABPA (37). These surprisingly high prevalence figures were generated from the ambulatory setting of the allergist-immunologist practice and suggest that the overall prevalence of ABPA in patients with persistent asthma is 1% to 2% (36). Allergic bronchopulmonary aspergillosis has been identified on an international basis (1,10,27,28,34,38), and because of its destructive potential should be confirmed or excluded in all patients with persistent asthma.
Aspergillus species are ubiquitous, thermotolerant, and can be recovered on a perennial basis (39,40). Spores (conidia) measure 2 μm to 3.5 μm and can be cultured on Sabouraud’s agar slants incubated at 37° to 40°C. Growth at this warm temperature is a somewhat unique property of Aspergillus fumigatus. Aspergillus hyphae may be identified in tissue by hematoxylin and eosin staining, but identification and morphology are better appreciated with silver methenamine or periodic acid-Schiff stains. Hyphae are 7 μm to 10 μm in diameter, septate, and classically branch at 45-degree angles. Aspergillus spores, which often are green, are inhaled from outdoor and indoor air and can reach terminal airways. They then could grow as hyphae. Airway epithelial cells phagocytose spores (41), but it is the alveolar macrophages that ingest and kill the spores (conidia) (41–43). Polymorphonuclear leukocytes do not ingest hyphae but bind to them and kill the hyphae by damaging their cell walls with a powerful, oxidative burst (41,44). Protection against invasive aspergillosis occurs due to multiple factors, but most crucial is the presence of functioning polymorphonuclear leukocytes because prolonged neutropenia (<500 cells/mm3) and possibly thrombocytopenia (as platelets bind to hyphae and become activated), injured pulmonary epithelium (from chemotherapy), insufficient local complement to facilitate opsonization and the oxidative burst by polymorphonuclear leukocytes, and overwhelmed innate immunity contribute to invasive disease.
Aspergillus species, particularly A. flavus and A. fumigatus, produce some toxic metabolites, of which aflatoxin is the most widely known. The measurement of aflatoxin is used to verify that foods, such as coffee beans and corn, are not contaminated. On a cellular level, a toxic and immunosuppressive metabolite, gliotoxin, inhibits macrophage phagocytosis and lymphocyte activation (41,45,46). A. fumigatus produces proteolytic enzymes and ribosome toxins (RNAses)
(41) that are thought to contribute to bronchial wall damage when A. fumigatus hyphae are present in bronchial mucus. Epithelial cells could be damaged by proteases from A. fumigatus that also would decrease ciliary function (40). Virulence factors generated by A. fumigatus include elastase, phospholipase, and acid phosphatase (47). In that cell membranes are composed of proteins and lipids, these enzymes could destroy the cell membranes and allow for unrestrained growth of spores and resultant damage to the bronchial wall (47). Also, surfactant is approximately 80% phospholipid, so that the phospholipases could interfere with normal lining fluid and immune responses to Aspergillus species (47).
(41) that are thought to contribute to bronchial wall damage when A. fumigatus hyphae are present in bronchial mucus. Epithelial cells could be damaged by proteases from A. fumigatus that also would decrease ciliary function (40). Virulence factors generated by A. fumigatus include elastase, phospholipase, and acid phosphatase (47). In that cell membranes are composed of proteins and lipids, these enzymes could destroy the cell membranes and allow for unrestrained growth of spores and resultant damage to the bronchial wall (47). Also, surfactant is approximately 80% phospholipid, so that the phospholipases could interfere with normal lining fluid and immune responses to Aspergillus species (47).
A. flavus and A. fumigatus have been incriminated in avian aspergillosis, a major economic concern in the poultry industry. For example, aspergillosis is common in turkey poults and can cause 5% to 10% mortality rates in production flocks (48). Aspergillus infections as a cause of abortions and mammary gland infections in sheep are recognized, as are infections in horses (pneumonia), cattle (pneumonia), camels (ulcerative tracheobronchitis), and dolphins (pneumonias including a condition resembling ABPA with cough, weight loss, and pulmonary infiltrates).
Aspergillus terreus is used in the pharmaceutical industry for synthesis of the cholesterol-lowering drug, levostatin. Aspergillus oryzae is invaluable in the production of soy products. Aspergillus niger is critical for production of citric acid. For use in the baking industry, Aspergillus species produce α-amylase, cellulase, and hemicellulase. Because these enzymes are powdered, some bakery workers may develop IgE-mediated rhinitis and asthma (49,50).
The genus Aspergillus may produce different types of disease, depending on the immunologic status of the patient. In nonatopic patients, Aspergillus hyphae may grow in damaged lung and cause a fungus ball (aspergilloma). Morphologically, an aspergilloma contains thousands of tangled Aspergillus hyphae in pulmonary cavities, and can complicate sarcoidosis, tuberculosis, old histoplasmosis, carcinoma, CF, or ABPA (51). Hypersensitivity pneumonitis may result from inhalation of large numbers of A. fumigatus or A. clavatus spores by malt workers. These spores also may produce farmer’s lung disease. Aspergillus species may invade tissue in the immunologically compromised (neutropenic and thrombocytopenic) host, causing sepsis and death. A rare patient, who seemingly is immunocompetent, may develop acute respiratory failure from bilateral “community acquired” pneumonia due to A. fumigatus infection. Aspergillus species have been associated with emphysema, colonization of cysts, pulmonary suppurative reactions, and necrotizing pneumonia in other patients (52,53). In the atopic patient, fungal spore–induced asthma may occur from IgE-mediated processes in response to inhalation of Aspergillus spores. About 25% of patients with persistent asthma have immediate cutaneous reactivity to A. fumigatus or a mix of Aspergillus species. Why some of these patients with asthma develop ABPA remains unclear. Genetic susceptibility includes HLA-DR2+, DRB*1501, and HLA-DQ2- as well as gain of function polymorphisms for IL-4 (54–56). In patients without asthma, Aspergillus hyphae have been identified in mucoid impactions of sinuses, a condition that morphologically resembles mucoid impaction of bronchi in ABPA (57–64). Such allergic Aspergillus rhinosinusitis may occur in patients with ABPA (26,27,65) (see Chapters 10 and 12).
There are over 185 Aspergillus species and additional variants. When A. fumigatus is grown in culture, changing media components and conditions alter the characteristics of the resultant strains of A. fumigatus. The International Union of Immunological Societies has recognized 22 allergens of A. fumigatus, which are listed as Asp f 1, 2, 3–18, 22w, 23, 27, 28, and 29 (see Chapter 6).
Diagnostic Criteria and Clinical Features
The criteria used for diagnosis of classic ABPA consist of five essential criteria and other criteria that may or may not be present, depending on the classification and stage of disease. The minimal essential criteria are (a) asthma, even cough-variant asthma or exercise-induced asthma; (b) central (proximal) bronchiectasis; (c) elevated total serum IgE (≥417 kU/L or 1,000 ng/mL); (d) immediate cutaneous reactivity to Aspergillus; and (e) elevated serum IgE and/or IgG antibodies to A. fumigatus. (66–68). Central (proximal) bronchiectasis in the absence of distal bronchiectasis, as occurs in CF or chronic obstructive pulmonary disease, is virtually pathognomonic for ABPA. Such patients are labeled ABPA-CB, for central bronchiectasis. Other features of ABPA are often present. For example, the expected diagnostic criteria (Table 24.1) of ABPA-CB include (a) asthma; (b) immediate cutaneous reactivity to A. fumigatus; (c) precipitating antibodies to A. fumigatus; (d) elevated total serum IgE concentration; (e) peripheral blood eosinophilia (≥1,000/mm3); (f) a history of either transient or fixed roentgenographic infiltrates; (g) proximal bronchiectasis; and (h) elevated serum IgE–A. fumigatus and IgG–A. fumigatus (66–68). These diagnostic criteria may not apply to ABPA-S (seropositive), where bronchiectasis cannot be detected by high-resolution chest tomography (12). Patients, who have all the criteria for ABPA but in whom proximal bronchiectasis is not present, have ABPA-S (12). The minimal essential criteria for ABPA-S include (a) asthma; (b) immediate cutaneous reactivity to Aspergillus; (c) elevated total serum IgE concentration; and (d) elevated serum IgE and IgG antibodies to A. fumigatus compared with sera from skin test positive patients with asthma without ABPA (12,67).
Table 24.1 Diagnostic Criteria for Allergic Bronchopulmonary Aspergillosis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Other features of ABPA may include positive sputum cultures for A. fumigatus and a history of expectoration of golden brown plugs containing A. fumigatus hyphae. Patients with asthma without ABPA may have positive cutaneous tests for A. fumigatus, peripheral blood eosinophilia, and a history of roentgenographic infiltrates (due to atelectasis from inadequately controlled asthma). Aspergillus precipitins are not diagnostic of ABPA, and sputum cultures may be negative for A. fumigatus or even unobtainable if the patient has little bronchiectasis. In ABPA-S, bronchiectasis cannot be detected by high-resolution CT. Serologic measurements have proven useful in making the diagnosis of ABPA. A marked elevation in total serum IgE concentration and IgE and IgG antibodies to A. fumigatus is of value in making the diagnosis (32,67–69). Furthermore, the decline in total serum IgE concentration by at least 35% by 6 weeks after institution of prednisone has been shown to occur in ABPA (70).
Allergic bronchopulmonary aspergillosis initially should be suspected in all patients with asthma who have immediate cutaneous reactivity to A. fumigatus (36,67). The absence of a documented chest roentgenographic infiltrate or mucoid infiltrates demonstrable by CT does not exclude ABPA-CB. Familial ABPA has been described occasionally, which emphasizes the need for screening family members for evidence of ABPA if they have asthma. Clearly, ABPA should be suspected in patients with a history of roentgenographic infiltrates, pneumonia, or abnormal chest films and in patients with allergic fungal rhinosinusitis. Increasing severity of asthma without other causes may indicate evolving ABPA, but some patients present solely with asymptomatic pulmonary infiltrates. Consolidation on the chest roentgenogram caused by ABPA often is not associated with the rigors, chills, as high a fever, and overall malaise as with a bacterial pneumonia causing the same degree of roentgenographic consolidation. The time of onset of ABPA may precede recognition by many years (72–74), or there may be early diagnosis of ABPA before significant lung destruction and roentgenographic infiltrates have occurred (12). Allergic bronchopulmonary aspergillosis must be considered in the patient over 40 years of age with chronic bronchitis, idiopathic bronchiectasis, or interstitial fibrosis. Further lung damage may be prevented by prednisone treatment of ABPA exacerbations. The dose of prednisone necessary for controlling persistent asthma may be inadequate to prevent the emergence of ABPA, although the total serum IgE concentration may be elevated only moderately because of suppression by prednisone.
Patients with ABPA manifest multiple allergic conditions. For example, only one of the initial 50 patients diagnosed and managed at Northwestern University Feinberg School of Medicine had isolated cutaneous reactivity to A. fumigatus (72). Other atopic disorders (rhinitis, urticaria, atopic dermatitis, drug allergy) may be present in patients with ABPA (72). The severity of asthma ranges from intermittent asthma to mildly persistent, to severe prednisone-dependent persistent asthma. Occasionally, patients deny developing wheezing or dyspnea on exposure to raked leaves, moldy hay, or damp basements, but they noted nonimmunologic triggering factors such as cold air, infection, or weather changes. The findings in these patients emphasize that ABPA may be present in patients who appear to have no obvious IgE-mediated asthma. Such patients can present with mucoid impactions and tenacious sputum and then have the diagnosis of ABPA made.
The number of diagnostic criteria vary depending on the classification (ABPA-CB or ABPA-S) and stage of ABPA. Furthermore, prednisone therapy causes clearing of the chest roentgenographic infiltrates, decline of the total serum IgE concentration, disappearance of precipitating antibodies, peripheral blood or sputum eosinophilia, and absence or reduction of sputum production.
Physical Examination
The physical examination in ABPA may be completely unremarkable in the asymptomatic patient, or the patient may have crackles, bronchial breathing, or wheezing, depending on the degree and quality of lung disease present. An acute exacerbation of ABPA may be
associated with temperature elevation to 103°F (although this is most uncommon), with malaise, dyspnea, wheezing, and sputum production. In some cases of ABPA, extensive pulmonary consolidation on roentgenography may be accompanied by few or no clinical symptoms, in contrast to the usual manifestations of a patient with a bacterial pneumonia and the same degree of consolidation. When extensive pulmonary fibrosis has occurred from ABPA, posttussive crackles will be present. Allergic bronchopulmonary aspergillosis has been associated with collapse of a lung from a mucoid impaction, and it was associated with a spontaneous pneumothorax (72). The physical examination yields evidence for these diagnoses. When ABPA infiltrates affect the periphery of the lung, pleuritis may occur and it may be associated with restriction of chest wall movement on inspiration and a pleural friction rub. Some patients with end-stage ABPA (fibrotic stage V) have digital clubbing and cyanosis (73,74). The latter findings should suggest concomitant CF as well.
associated with temperature elevation to 103°F (although this is most uncommon), with malaise, dyspnea, wheezing, and sputum production. In some cases of ABPA, extensive pulmonary consolidation on roentgenography may be accompanied by few or no clinical symptoms, in contrast to the usual manifestations of a patient with a bacterial pneumonia and the same degree of consolidation. When extensive pulmonary fibrosis has occurred from ABPA, posttussive crackles will be present. Allergic bronchopulmonary aspergillosis has been associated with collapse of a lung from a mucoid impaction, and it was associated with a spontaneous pneumothorax (72). The physical examination yields evidence for these diagnoses. When ABPA infiltrates affect the periphery of the lung, pleuritis may occur and it may be associated with restriction of chest wall movement on inspiration and a pleural friction rub. Some patients with end-stage ABPA (fibrotic stage V) have digital clubbing and cyanosis (73,74). The latter findings should suggest concomitant CF as well.
Radiology
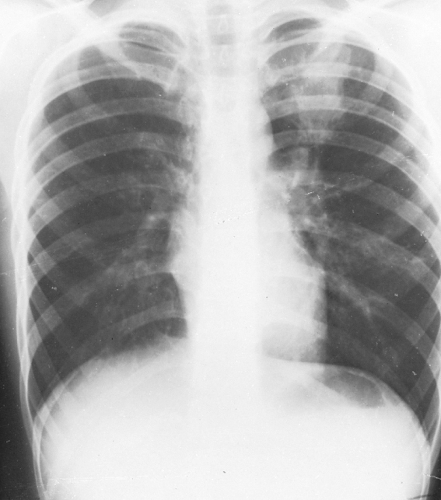
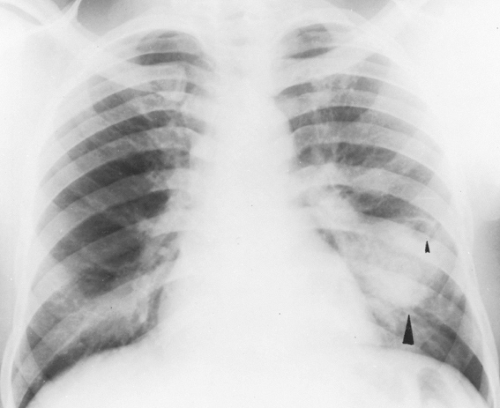
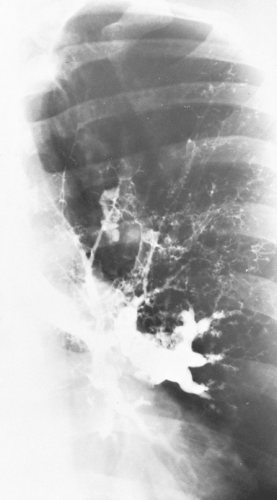
Chest roentgenographic changes may be transient or permanent (Figs. 24.1 to 24.6) (75,76). Transient roentgenographic changes, which may clear with or without oral corticosteroid therapy, appear to be the result of parenchymal infiltrates, mucoid impactions, or secretions in damaged bronchi (9,10,75–81). These nonpermanent findings include (a) perihilar infiltrates simulating adenopathy; (b) air-fluid levels from dilated central bronchi filled with fluid and debris; (c) massive consolidation that may be unilateral or bilateral; (d) roentgenographic infiltrates; (e) “toothpaste” shadows that result from mucoid impactions in damaged bronchi; (f) “gloved-finger” shadows from distally occluded bronchi filled with secretions; and (g) “tramline” shadows, which are two parallel hairline shadows extending out from the hilum. The width of the transradiant zone between the lines is that of a normal bronchus at that level (75). Tramline shadows, which represent edema of the bronchial wall, may be seen in asthma without ABPA, in CF, and in left ventricular failure with elevated pulmonary venous pressure. Permanent roentgenographic findings related to proximal bronchiectasis have been shown to occur in sites of previous infiltrates, which are often, but not exclusively, in the upper lobes. This is in contrast to postinfectious bronchiectasis, which is associated with distal abnormalities and normal proximal bronchi. When permanent lung damage occurs to large bronchi, parallel line shadows
and ring shadows are seen. These do not change with oral corticosteroids. Parallel line shadows are dilated tramline shadows that result from bronchiectasis; the transradiant zone between the lines is wider than that of a normal bronchus. These shadows are believed to be permanent, representing bronchial dilation. The ring shadows, 1 cm to 2 cm in diameter, are dilated bronchi en face. Pulmonary fibrosis may occur and might be irreversible. Late findings in ABPA include cavitation, contracted upper lobes, fibrosis, and localized emphysema. When bullous changes are present, a spontaneous pneumothorax may occur (72).
and ring shadows are seen. These do not change with oral corticosteroids. Parallel line shadows are dilated tramline shadows that result from bronchiectasis; the transradiant zone between the lines is wider than that of a normal bronchus. These shadows are believed to be permanent, representing bronchial dilation. The ring shadows, 1 cm to 2 cm in diameter, are dilated bronchi en face. Pulmonary fibrosis may occur and might be irreversible. Late findings in ABPA include cavitation, contracted upper lobes, fibrosis, and localized emphysema. When bullous changes are present, a spontaneous pneumothorax may occur (72).
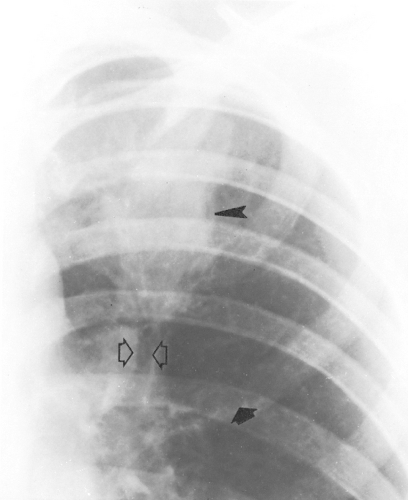
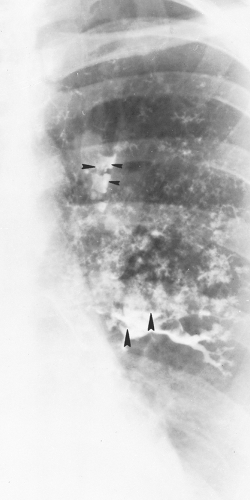
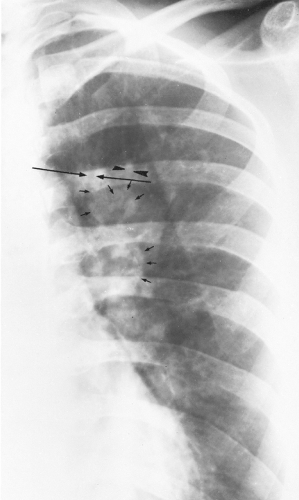 Figure 24.6 Magnified view of the left upper lung of the patient shown in Figs. 24.4 and 24.5 demonstrates parallel lines (long arrows) and toothpaste shadows (arrowheads). Perihilar infiltrates (pseudohilar adenopathy) and a gloved-finger shadow also are seen (small arrows). (Reprinted from Mintzer RA, Rogers LF, Kruglick GD, et al. The spectrum of radiologic findings in allergic bronchopulmonary aspergillosis. Radiology 1978;127:301; with permission.) |
With high clinical suspicion of ABPA (bronchial asthma, high total serum IgE concentration, immediate cutaneous reactivity to A. fumigatus, precipitating antibody against A. fumigatus) and a negative chest roentgenogram, central bronchiectasis may be demonstrated by high-resolution computed tomography (CT) (77–81). This examination should be performed as an initial radiologic test beyond the chest roentgenogram (Figs. 24.7
through 24.9). If findings are normal, studies should be repeated in 1 to 2 years for highly suspicious cases.
through 24.9). If findings are normal, studies should be repeated in 1 to 2 years for highly suspicious cases.
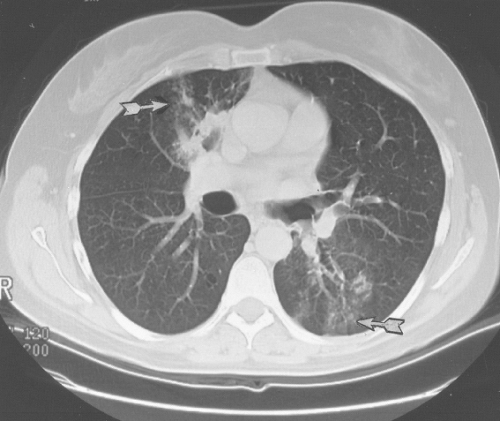
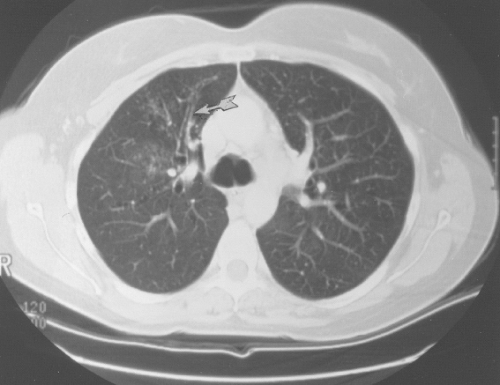 Figure 24.8 Dilated bronchi from an axial longitudinal orientation (arrow) consistent with bronchiectasis (same patient as in Fig. 24.7). |
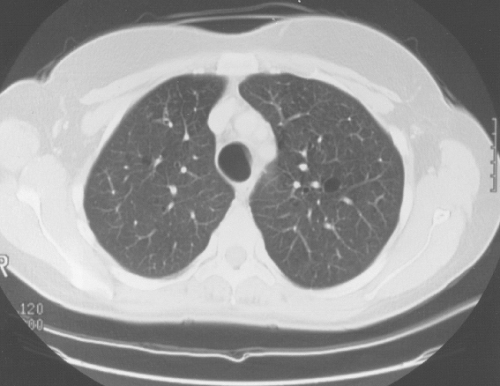 Figure 24.9 Cystic (dilated) bronchi and bronchioles (same patient as in Fig. 24.7). |
High-resolution CT using 1.5-mm section cuts has proved valuable in detecting bronchiectasis in ABPA (77–81). The thin-section cuts were obtained every 1 cm to 2 cm from the apex to the diaphragm. The use of high-resolution CT examinations has identified areas of cylindrical bronchiectasis in patients with asthma. However, the areas are localized and the patients do not have sufficient other criteria to make the diagnosis of ABPA. For example, bronchial dilatation was present in 41% of lung lobes in eight ABPA patients compared with 15% of lobes in patients with asthma without ABPA. Bronchiectasis may be cylindrical, cystic, or varicose (77–79). From the axial perspective, proximal bronchiectasis is present when it occurrs in the inner two-thirds of the lung.
When high-resolution CT using 1 mm to 3 mm of collimation (thin sections) was performed in 44 patients with ABPA and compared with 38 patients with asthma without ABPA, bronchiectasis was identified in both
patient groups (79). Bronchiectasis was present in 42 (95%) ABPA patients compared with 11 (29%) patients with asthma. The CT scans revealed bronchiectasis in 70% of lobes examined in ABPA versus 9% of lobes from patients with asthma (79). Some 86% of ABPA patients had three or more bronchiectatic lobes, whereas 91% of the patients with asthma had bronchiectasis in one or two lobes. In the ABPA patients, bronchiectasis was varicose in 41% of patients, cystic in 34% of patients, and cylindrical in 23% of patients. Consolidation was identified in 59% of ABPA patients, primarily being located peripherally, whereas consolidation was present in 9% of patients with asthma (79).
patient groups (79). Bronchiectasis was present in 42 (95%) ABPA patients compared with 11 (29%) patients with asthma. The CT scans revealed bronchiectasis in 70% of lobes examined in ABPA versus 9% of lobes from patients with asthma (79). Some 86% of ABPA patients had three or more bronchiectatic lobes, whereas 91% of the patients with asthma had bronchiectasis in one or two lobes. In the ABPA patients, bronchiectasis was varicose in 41% of patients, cystic in 34% of patients, and cylindrical in 23% of patients. Consolidation was identified in 59% of ABPA patients, primarily being located peripherally, whereas consolidation was present in 9% of patients with asthma (79).
Staging
Five stages of ABPA have been identified (11). These stages are acute, remission, exacerbation, corticosteroid-dependent asthma, and fibrotic. The acute stage (stage I) is present when all the major criteria of ABPA can be documented. These criteria are asthma, immediate cutaneous reactivity to A. fumigatus




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree