ALDOSTERONE-PRODUCING ADENOMAS
Part of “CHAPTER 80 – HYPERALDOSTERONISM“
CLINICAL FEATURES AND PATHOPHYSIOLOGY
Aldosterone-producing adrenocortical adenomas usually are small (0.5–2.5 cm), unilateral, solitary, and associated with hypoplastic zona glomerulosa. Occasionally, however, these lesions may be multiple and associated with hyperplasia of the zona glomerulosa. This benign tumor of the adrenal cortex occurs most frequently in the third through the fifth decades of life but also has been observed in prepubertal children6 and older persons (range, 13–66 years). Aldosterone-producing adenomas occur equally in men and women and do not appear to have a racial predisposition; they tend to develop more frequently in the left adrenal gland than in the right, but this difference is small.
(The occurrence of primary aldosteronism resulting from APA in two or more members of the same family has been reported,7 as well as the familial occurrence of idiopathic aldo-steronism. In one of the families, one member had bilateral nodular hyperplasia and another had APA. This familial form of primary aldosteronism was labeled familial hyperaldosteronism type II to distinguish it from glucocorticoid-remediable hyperaldosteronism. The clinical features of familial hyperal-dosteronism type II do not differ from those of sporadic patients with APA or idiopathic hyperaldosteronism.)
The symptoms reported most frequently by patients with an APA are headache, easy fatigability, and weakness, although high blood pressure may be the only symptom in many cases. Hypokalemia, suppressed plasma renin activity, and high plasma and urinary aldosterone values are the findings usually associated with APA, but they may not always be demonstrable.8
Aldosterone production by an APA is autonomous and is affected only minimally by angiotensin II because of the decreased formation of this peptide as well as the decreased responsiveness of the adenoma. An APA is unresponsive to the infusion of dopamine, although endogenous dopamine may exert tonic inhibitory effects.9 ANH, levels of which appear to be elevated in patients with this disorder,10 does not inhibit aldosterone biogenesis by APA cells in vitro, as it does in normal glomerulosa cells.11 Therefore, this hormone may have little effect on aldosterone secretion by an APA in vivo. In contrast, changes in serum potassium or ACTH levels may exert important regulatory effects on aldosterone secretion in patients with this tumor: aldosterone production may be blunted by the potassium loss and the hypokalemia that it induces. The circadian rhythm of aldosterone in patients with APA tends to mirror that of ACTH.
As a consequence of sustained overproduction of aldosterone, retention of sodium chloride and water occurs, but this usually is limited; “escape” occurs when extracellular fluid has been expanded by ˜500 mL.12 Initially, the renal excretion of potassium increases. As hypokalemia develops, potassium excretion decreases to reflect intake of this cation. Hydrogen ion excretion, which increases principally because of an increase in urinary ammonium, is responsible for the development and maintenance of metabolic alkalosis. Urinary calcium and magnesium levels are high; presumably, this is a reflection of the effects of expansion of extracellular fluid on the tubular reabsorption of these ions. The urinary concentrating ability is impaired because of potassium depletion. Although sodium and water retention may contribute to the increase in blood pressure that occurs, the precise mechanisms responsible for an increase in peripheral resistance and the associated hypertension are unclear. Receptors for aldosterone have been found in blood vessels and in the brain, and the effects of aldosterone on ion transport in these tissues may be important mediators of the increase in blood pressure. Preliminary studies in dogs indicate that an amount of aldosterone that is too small to exert an effect when infused peripherally increases blood pressure when it is infused into the third ventricle of the brain.13
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
Patients with hypertension who have borderline or low serum potassium concentrations, low stimulated plasma renin activity (<2 ng/mL per hour), and borderline or high plasma aldosterone concentrations (>14 ng/dL) should be evaluated further for autonomous overproductionof aldosterone.
The evaluation should be done before antihypertensive treatment is instituted or after it has been discontinued for 2 weeks. Determining aldosterone suppressibility, by combining a high sodium intake and treatment with a mineralocorticoid such as 9-αfluorohydrocortisone for 3 or more days, or by intravenous saline infusion, is a useful first step. Of these two interventions, the infusion of 2 L normal saline over 4 hours with the measurement of plasma aldosterone levels before and at the conclusion of the infusion is simpler and may be readily adapted to the evaluation of outpatients.14 At the conclusion of the infusion, the plasma aldosterone level normally is 5 ng/dL or less; in patients with hyperaldosteronism, it usually exceeds 10 ng/dL.
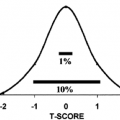
Determination of the plasma aldosterone to plasma renin activity ratio also has been used to detect aldosteronism. Single determinations of this ratio tend to be of limited use because values from patients with hyperaldosteronism frequently are the same as those from patients with normal adrenal function. A solution to the problem of overlap that appears to improve specificity considerably is to collect blood samples every 30 minutes for 6 hours, so that an integrated plasma aldosterone/plasma renin activity ratio can be determined.15 A somewhat simpler but probably equally effective alternative is to collect blood 90 minutes after the administration of 25 to 50 mg captopril, which is a converting enzyme inhibitor, for determination of the plasma aldosterone/plasma renin activity ratio16 (Fig. 80-3). Both procedures are safe and do not require dietary preparation or hospitalization. It has been suggested that all patients with hypertension undergo captopril screening tests,17 because determination of the serum potassium concentration and plasma renin activity may be inadequate measures for screening the hypertensive population for hyperaldosteronism.8 The normogram in Figure 80-3 indicates the degree of separation of patients with aldosterone excess from those with essential hypertension.16 Subsequent evaluation of the captopril test indicates a false-negative rate of 6.3% and a false-positive rate of 0.6% in patients with primary hyperaldosteronism.18 The problem of identifying those patients with hyperaldosteronism who harbor an adenoma has been greatly facilitated by technologic advances. Computed tomography (CT) appears to have the capacity to discern an adrenal mass in ˜80% of patients with an aldosterone-producing adenoma.19,20 Magnetic resonance imaging also has been used to evaluate the adrenal glands in patients with hyperaldosteronism; apart from confirming the findings of CT, however, it does not appear to offer any advantage over CT. These new techniques for imaging adrenal masses have replaced the more cumbersome and time-consuming procedure of131 Iiodocholesterol imaging in most centers (see Chap. 88). A limitation of all the imaging techniques, however, is that an adenoma may be poorly visualized or not detected at all. These problems result from the small size of some adenomas, which may be less than 1 cm in diameter. The problem
of an inconclusive CT scan may be partially resolved by determining the plasma 18-hydroxycorticosterone level after overnight bedrest.21 In a series of 50 patients with aldosteronism, all but 1 of the 24 patients with an adenoma had 18-hydroxycorticosterone values that exceeded 50 ng/dL.8 Thus, a plasma 18-hydroxycorticosterone value >50 ng/dL in a patient with hyperaldosteronism suggests that the diagnosis is more likely to be an aldosterone-producing adenoma than bilateral hyperplasia, even though the adrenal glands may appear normal (Fig. 80-4). If the plasma aldosterone level were to show a “paradoxic” fall paralleling the circadian fall in plasma cortisol during 2 hours of ambulation after early morning sampling, this also would suggest that the diagnosis is more likely to be an aldosterone-producing adenoma than bilateral hyperplasia.22 So many patients with an adenoma, like those with bilateral hyperplasia, show a rise rather than a fall in the plasma aldosterone level that the usefulness of the procedure is limited.8
of an inconclusive CT scan may be partially resolved by determining the plasma 18-hydroxycorticosterone level after overnight bedrest.21 In a series of 50 patients with aldosteronism, all but 1 of the 24 patients with an adenoma had 18-hydroxycorticosterone values that exceeded 50 ng/dL.8 Thus, a plasma 18-hydroxycorticosterone value >50 ng/dL in a patient with hyperaldosteronism suggests that the diagnosis is more likely to be an aldosterone-producing adenoma than bilateral hyperplasia, even though the adrenal glands may appear normal (Fig. 80-4). If the plasma aldosterone level were to show a “paradoxic” fall paralleling the circadian fall in plasma cortisol during 2 hours of ambulation after early morning sampling, this also would suggest that the diagnosis is more likely to be an aldosterone-producing adenoma than bilateral hyperplasia.22 So many patients with an adenoma, like those with bilateral hyperplasia, show a rise rather than a fall in the plasma aldosterone level that the usefulness of the procedure is limited.8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree