Fig. 6.1
Computerized tomography of a benign adrenocortical adenoma of the left adrenal (arrow). Reprinted with permission from Androulakis et al. [19]
Arterial hypertension (AH) is a prevailing, but not an innocent bystander in the aging population. The prevalence of AH increases with advanced age [13]. Several studies have reported an increased prevalence of arterial hypertension, diabetes mellitus and/or glucose intolerance, hyperlipidemia, and obesity in AI patients, reaching up to 54%, 66%, 36%, and 28%, respectively [14–18]. The reported incidence of AH in studies investigating patients with AI ranges between 36 and 67% and is cumulatively depicted in Table 6.1. However, there is a wide variation in the reported prevalence due to the different inclusion criteria, methodology used, and cut-offs employed.
Table 6.1
Cumulative data on the prevalence of arterial hypertension in studies of patients harboring an adrenal incidentaloma
First author | Study population | Prevalence of AH Number of patients (%) | References |
Fernandez-Real, Clin Endocrinol 1998 | NFAI | 20/64 (31) | [14] |
Barzon, JCEM 1999 | AI | 41/75 (55) | [15] |
Mantero, JCEM 2000 | AI | 362/863 (41) | [16] |
SCS | 38/92 (41) | ||
Rossi, JCEM 2000 | AI | 24/50 (48) | [17] |
SCS | 11/12 (92) | ||
Midorikawa, Clin Endocrinol 2001 | AI | 7/12 (58) | [27] |
Tauchmanovà, JCEM 2002 | SCS | 17/28 (61) | [28] |
Terzolo, Eur J Endocrinol 2005 | AI | 113/210 (54) | [30] |
SCS | 8/23 (35) | ||
Erbil, World J Surg 2006 | SCS | 7/11 (63) | [31] |
Comlekci, Endocrine 2010 | AI | 207/376 (55) | [32] |
Giordano, Eur J Endocrinol 2010 | AI | 73/118 (61) | [33] |
NFAI | 59/102 (58) | ||
SCS | 13/16 (81) | ||
Terzolo, Clin Endocrinol 1998 | AI | 19/53 (36) | [37] |
Piaditis, Clin Endocrinol 2009 | AI | 41/83 (49) | [39] |
Libè, Eur J Endocrinol 2002 | AI | 35/64 (55) | [44] |
Bernini, Br J Cancer 2005 | AI | 76/115 (66) | [45] |
Vassilatou, Clin Endocrinol 2009 | NFAI | 32/57 (56) | [47] |
SCS | 15/20 (75) | ||
Reincke, JCEM 1992 | SCS | 7/8 (88) | [49] |
Bernini, Eur J Endocrinol 2003 | AI | 10/15 (67) | [50] |
SCS | 5/6 (83) | ||
Mitchell, Surgery 2007 | SCS | 8/9 (89) | [51] |
Tsuiki, Endocr J 2008 | SCS | 9/20 (45) | [52] |
Sereg, Eur J Endocrinol 2009 | NFAI | 93/113 (82) | [53] |
Chiodini, JCEM 2010 | AI | 62/108 (57) | [54] |
SCS | 24/41 (59) | ||
Toniato, Ann Surg 2009 | SCS | 33/45 (73) | [55] |
Wagnerova, Neoplasma 2009 | AI | 51/92 (55) | [102] |
Yilmaz, J Endocrinol Invest 2009 | NFAI | 6/32 (20) | [103] |
The adverse metabolic and cardiovascular profile observed in AI has initiated the search for potential links between AH and other potentially adverse metabolic parameters [15, 18, 19]. This is reinforced further by the presence of surrogate markers of cardiovascular dysfunction even in patients with apparently nonfunctioning AI. Such patients have worse indices of left ventricle hypertrophy and diastolic function compared to normotensive lean subjects, whereas hypertensive AI patients have the most impaired cardiac morphology and function [20]. Additionally, patients with nonfunctioning AI have significantly lower flow-mediated dilation, a marker of endothelial function, compared to age, sex, and body mass index (BMI)-matched control group [21]. Furthermore, early atherosclerotic changes such as increased carotid intima-media thickness are found in nonfunctioning AI subjects [22].
The scope of this chapter is to provide an update on current literature concerning the relation of arterial hypertension with AI.
Subclinical Cushing’s syndrome
Cushing’s syndrome (CS) is accompanied by increased morbidity and mortality attributed mostly to a higher cardiovascular disease (CVD) risk. Among other metabolic features, AH is frequently found in subjects with CS, especially in adults (80%), and less commonly in the pediatric population [23–25]. Even in subclinical cases, the incidence of AH in AI-related subclinical Cushing’s syndrome (SCS) is high in several clinical reports varying from 35% up to 92% [16–18, 26–33]. Overt or subtle hypercortisolism is associated with hypertension through the activation of the mineralocorticoid receptor, following saturation of the 11 beta-hydroxysteroid dehydrogenase 2 (11βHSD2) enzyme, that inactivates cortisol to cortisone in the renal cortex (see below). SCS refers to a state of altered hormonal secretory dynamics and autonomous glucocorticoid production without apparent clinical signs and symptoms. The term “subclinical” is preferred to “preclinical,” since the majority of cases do not develop overt Cushing’s syndrome (CS) after prolonged follow-up. The absence of Cushing’s phenotype rests in clinical judgment, but even for experienced clinicians it may be difficult to attribute a sign to hypercortisolism or the other encountered entities such as the metabolic syndrome (MS). SCS is the most common finding when evaluating an incidentally discovered adrenal mass and its prevalence has been reported to vary from 5 to 56%, but its clinical significance is not as yet fully defined [8, 17, 29, 34–39]. Biochemical tests applied to diagnose hypercortisolism include 24-h urinary-free cortisol (UFC), midnight salivary cortisol, and dexamethasone suppression test (DST). Lowering cortisol cut-off levels following the DST, the proposed screening test in AI, is anticipated to reveal more cases of SCS. Measurement of ACTH and dehydroepiandrosterone (DHEAS) levels has also been proposed, but the ideal strategy to detect SCS in AI is not yet established and controversy exists between European and American Societies [40, 41]. Recently, it was suggested that the cost-effectiveness of extensive evaluation for SCS should be reappraised [42]. Diagnostic algorithms for Cushing’s syndrome have been published by the Endocrine Society and are explicitly reviewed in the relevant book chapter [43].
Current literature suggests that progression of SCS to overt Cushing’s syndrome is uncommon, whereas occasionally resolution of functional alterations has also been observed [8, 15, 17, 37, 44–48]. Patients with SCS appear to harbor an unfavorable metabolic profile with increased frequency of arterial hypertension, diabetes mellitus, central adiposity, and dyslipidemia [16–18, 26–33]. This raises the question whether surgical management is indicated in AI bearing subtle cortisol overproduction. It should be stressed, however, that there is scant data supporting the increased CVD morbidity and mortality in AI patients exhibiting SCS. These associations have favored the implementation of surgical therapy that led to some improvement of AH and other metabolic parameters in SCS patients. However, as long-term results are lacking, definite conclusions are probably arbitrary [15, 17, 29, 31, 33, 49–54]. In the only randomized trial up to now, Toniato et al. demonstrated a significant improvement of AH in the patients’ group treated surgically compared to those managed conservatively [55]. In that study, 12 out of 18 surgically treated SCS patients (67%) exhibited stabilization or improvement of their blood pressure control, whereas in the conservatively managed group 12 out of 15 hypertensives required change or increase of their antihypertensive medication. However, the final therapeutic approach is based on an individualized basis, taking into consideration the surgical risk, expense, and outcome of laparoscopic adrenalectomy compared to life-style modification and pharmacological intervention. Some advocate that the surgical approach should be employed for young patients with AI associated with an unfavorable metabolic profile that is unresponsive to medical treatment [4, 41]. In case of surgical management, postoperative glucocorticoid coverage is used until recovery of the adrenal function [56]. In every case, surgical or medical management of hypercortisolism is necessary in order to lower or—when possible—normalize blood pressure and thus ameliorate the CVD risk profile of patients with CS.
Pathophysiological Link Between Hypercortisolism and AH
The “dipping” phenomenon, the fall of blood pressure at nighttime, was suggested to be the link between hypercortisolism and AH, as it was markedly lower in CS compared to subjects with essential hypertension without CS. Furthermore, this abnormality was resolved with the treatment of CS [57]. As previously stated, excessive cortisol production in CS results in substrate saturation of the 11βHSD2 enzyme, responsible for the inactivation of cortisol to cortisone in the renal cortex, but also in vascular endothelial cells and vascular smooth muscle cells [58]. This results in spillover of cortisol and binding with the mineralocorticoid receptor, thus inducing sodium reabsorption from the renal tubule, volume expansion, and finally hypertension [24]. However, spironolactone administration, a mineralocorticoid receptors antagonist, did not prevent the development of hypertension [59]. Therefore, cortisol-induced hypertension cannot be solely interpreted by the mineralocorticoid effect of cortisol and it is likely that other factors may also be implicated [60].
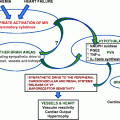
According to animal data, glucocorticoids enhance angiotensin II production and up-regulate its receptors in both the brain and periphery [61, 62]. Besides activation of the renin–angiotensin–aldosterone system (RAAS), it appears that glucocorticoids decrease vasodilation by inhibiting the production of nitric oxide (NO) and prostacyclin [63]. Defective transport of l-arginine, a precursor of NO synthesis, as well as inhibition, uncoupling, and increased inactivation of NO synthase are thought to participate in cortisol-induced hypertension [64]. Glucocorticoid-induced oxidative stress hypertension has also been implicated through the stimulation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase pathway and an imbalance between reactive oxygen species (ROS) production and degradation [64]. Vasoconstrictive substances, such as endothelin-1 and erythropoietin, are also elevated in hypercortisolism states [65, 66]. Given the discrepancy of animal and human data, further studies are warranted to identify the exact role of these substances. Moreover, clinical studies in healthy subjects have demonstrated that glucocorticoids enhance the sensitivity of the vasculature to catecholamines, induce peripheral vascular resistance, and increase arterial blood pressure [67]. Figure 6.2 illustrates a summary of the underlying mechanisms in cortisol-induced hypertension.
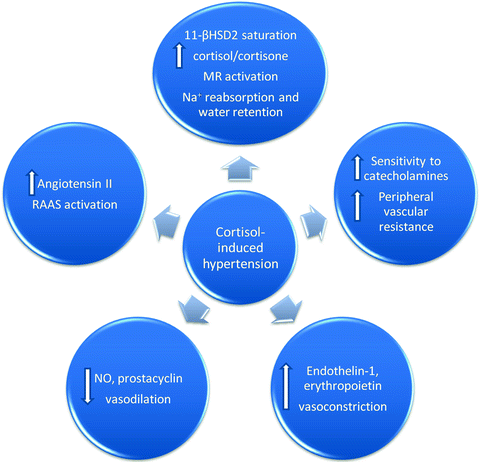

Fig. 6.2
Proposed pathogenetic mechanisms of cortisol-induced hypertension. 11-βHSD2 11-beta-hydroxysteroid dehydrogenase type 2; MR mineralocorticoid receptor; Na + sodium; RAAS renin–angiotensin–aldosterone system; NO nitric oxide
Primary Aldosteronism: Autonomous Aldosterone Secretion
Primary aldosteronism (PA) is the syndrome resulting from autonomous aldosterone secretion (AAS) leading to hypokalemia, endothelial dysfunction, arterial stiffness, cardiovascular hypertrophy, and fibrosis placing PA patients at a significant CVD risk [68, 69]. The vast majority of cases are due to an aldosterone-producing adrenal adenoma (APA) and bilateral adrenal hyperplasia or idiopathic PA (IPA). The differential diagnosis is crucial, since APA is cured with unilateral adrenalectomy, whereas the treatment of choice for IHA includes antihypertensive medication targeting the RAAS. Early diagnosis and tailored treatment is pivotal in the prevention or regression of CVD alterations induced by aldosterone overproduction.
In adult hypertensives the prevalence of PA is estimated to be approximately 13% [70], whereas its prevalence is increased with the severity of AH [71]. According to the Primary Aldosteronism Prevalence in Hypertensives (PARY) Study, the prevalence of PA was 11.2% with APA comprising 43% of the cases [72]. PA appears to be the most frequent cause of endocrine AH and, as a result, all hypertensive patients diagnosed with an AI should undergo testing for PA. The initial diagnostic tool to screen for PA is the aldosterone/renin ratio (ARR), i.e., the ratio of plasma aldosterone levels (ng/dL) divided by the plasma renin activity (PRA) (ng/mL/h). The most widely used cut-off values are 20–30 [73]. However, sensitivity of the test ranges widely mainly due to the PRA induced wide variation of ARR [74]. It has therefore been suggested that clinicians do not rely solely to a single ARR to diagnose patients with suspected PA [70]; and that repeated measurements of the ARR should be obtained [75]. The oral saline loading, the saline infusion, the fludrocortisone with salt loading test, and the captopril challenge test are second-line tests used to confirm or exclude the presence of PA. Guidelines for the diagnosis of PA have been issued by Endocrine Society [73] and a concise update on the diagnosis and treatment of PA is included in a previous chapter of this book, where the reader is referred for a detailed literature review.
The prevalence of PA in patients bearing an AI has been estimated to range from 1 up to 24% depending on the hormonal test used to diagnose PA [16, 26, 34, 36, 39, 76–79]. However, it should be acknowledged that the cut-off value of the screening and the confirmatory test exploited in the diagnostic process is a major determinant of the estimated prevalence of PA and an important guide to further testing. We have recently found an increased prevalence of AAS among hypertensive patients (31%) and in subjects with unilateral adrenal adenomas (24%) [39, 80]. Similar results were found when investigating patients with incidentally discovered bilateral adrenal masses [81]. This higher than previously described prevalence of PA was attributed to the methodology used as cut-off values were obtained from normotensive subjects with normal adrenal morphology [39]. Furthermore, the confirmatory saline infusion test of PA was modified with prior dexamethasone administration to eliminate the effect of adrenocorticotropin hormone (ACTH) on aldosterone secretion [80]. The above features underline the need to take into consideration the methodology used when interpreting study findings on the prevalence of PA. The clinical significance of subtle AAS forms remains unknown, but data from the Framingham Offspring showed that normotensive subjects with high aldosterone levels, yet in the normal range, were at increased risk for the development of AH [82].
Pathophysiological Mechanism of Aldosterone-Induced Hypertension
Aldosterone is well known to act via the mineralocorticoid receptor to promote renal sodium retention. Additionally, it augments angiotensin II activity and can thus lead to endothelial dysfunction and impaired vascular resistance. One should also consider the significant paracrine actions of aldosterone in the central nervous system, the myocardium, and the brain independent of the circulating aldosterone levels [83–85]. It is possible that these mechanisms may mediate an alternative pathway to aldosterone-induced hypertension.
Pheochromocytoma
Pheochromocytomas (PHEO), tumors arising from catecholamine-producing chromaffin cells in the adrenal medulla, are an uncommon cause of AH [86]. The classical constellation includes hypertension, headaches, palpitations, and diaphoresis. Timely diagnosis of this “great mimicker” and effective treatment is critical in order to avoid or attenuate the long-term consequences on the cardiovascular, cerebrovascular, and renal system. Besides imaging studies, biochemical screening tests include blood and urine measurements of catecholamines and their metabolic products (adrenaline, noradrenaline and metanephrine, normetanephrine and vanillylmandelic acid, respectively) with fractionated metanephrine and normetanephrine being the preferred initial choice [87].
PHEO may be a rare cause of AH, but when occurs the vast majority of patients have AH related to excessive catecholamine production. AH may be sustained or paroxysmal (50% and 45%, respectively) and occasionally patients may have paroxysms of AH in a setting of sustained hypertension. Until recently, only few patients with PHEO (5–15%) were considered to have normal arterial pressure [88]. Several factors influence the phenotypic characteristics of AH in patients with PHEO, such as the amount of catecholamines produced by the tumor and their secretory pattern [89]. Excessive norepinephrine secretion is considered to induce vasoconstriction, thus resulting in sustained hypertension. Epinephrine hypersecretion is commonly associated with paroxysmal hypertension and is more frequently encountered in PHEO patients with multiple endocrine neoplasia 2 (MEN-2) syndromes [89]. Albeit a minority, patients harboring a very small, familial, or dopamine-producing PHEO may have normal arterial pressure. These subjects represent a special, not negligible, subgroup of PHEO patients with atypical symptoms that may be diagnosed in the evaluation of an incidentally discovered adrenal mass.
Lately, due to increased awareness and with the advent of imaging techniques, the prevalence of incidentally identified, “subclinical” PHEO is rising [8, 16, 26, 35, 90–97]. A PHEO prevalence of 10% has been reported among patients undergoing operation for an adrenal incidentaloma [98]. In an Italian study, PHEO were found to comprise 3.4% of AIs and half of these incidentally diagnosed PHEO had no elevations of blood pressure [16]. Lee et al. found borderline elevation of urinary or plasma metanephrine levels in 24% of patients with an AI, and among them, one third were diagnosed to have subclinical or asymptomatic PHEO according to pathological examination [99]. The authors proposed repeating biochemical testing along with iodine-131-meta-iodobenzylguanidine (MIBG) scanning or preoperative alpha-blockade in AI subjects with marginal elevation of metanephrine levels. In alignment with the previous studies, in a German cohort of 201 PHEOs, almost 30% of them were incidental findings confirming the need for evaluation of PHEO in all AI patients [100]. In a further study, incidental discovery of a PHEO was associated with older age and lower catecholamine concentrations compared to symptomatic patients [95], in agreement with the findings of Grozinsky-Glasberg et al. in their study in clinically silent PHEOs [101].
Nonfunctioning Adrenal Incidentalomas
The majority of AI is not hormonally active, but increasing evidence is emerging concerning an increased CVD risk in patients with NFAIs. The prevalence of AH, diabetes mellitus, fatty liver disease, and dyslipidemia is higher in NFAI subjects compared to the general population [102, 103] whereas recently an increased prevalence of insulin resistance in NFAIs was reported [104]. However, it should be stressed that the classification of AI as nonfunctioning relies on the cut-off values applied in their hormonal evaluation. It is highly probable that lowering the cut-offs used to identify autonomous adrenal steroid production will identify more AI with subtle hormonal overproduction and decrease the prevalence of the up to now recognized NFAIs. The beneficial effect of adrenalectomy in NFAIs on AH and other metabolic markers demonstrated in two studies may suggest that NFAIs harbor an increased CVD risk [17, 50]. However, controversy exists as a further study showed no improvement in blood pressure control and no difference in cardio- and cerebrovascular morbidity [53].
The design of the clinical studies evaluating the metabolic status of AI patients and the inconsistency of data from studies evaluating the outcome of adrenalectomy does not permit the optimal characterization of the relation between AI and the parameters of the metabolic syndrome as causal. An interesting approach was introduced by Reincke et al., who showed a mitogenic effect of insulin on adrenal cancer cells in vitro and proposed that sustained hyperinsulinemia may lead to adrenocortical tumorigenesis [105]. The therapeutic dilemmas in AI can only be solved with longitudinal, properly controlled studies that will assess the evolution of various metabolic parameters, like AH, diabetes, hyperlipidemia, but most importantly should address the role of adrenalectomy—if any—in “hard” outcomes, like major CVD events and mortality.
Conclusions
SCS is the most frequently encountered hormonal abnormality when evaluating a patient with an AI and appears to follow a relatively indolent course without progression to overt disease. The association of hypertension with SCS is very strong and consistent. In most, but not all, studies a marked improvement of AH is observed following surgical treatment, but this cannot at the present time be translated in reduction of CVD risk. Therefore, the ideal management of SCS patients, surgical or pharmacological, remains a debate. Primary aldosteronism is considered the most common cause of endocrine hypertension. The emerging data on the role of aldosterone in the heart and the vasculature renewed the interest in PA. Controversy exists regarding the suitable screening test and its cut-off value that will not miss patients with AAS yet not at the cost of a high false-positive number. Concerning pheochromocytomas, a change in their initial presentation is reported with a growing number of these chromaffin-cell tumors being discovered incidentally, the so-called silent pheochromocytomas. Early identification and establishing the correct diagnosis in these borderline cases is critical for the preoperative medical blockade of the patients to prevent a life-threatening hypertensive crisis intra-operatively. The clustering of parameters of the metabolic syndrome, like AH, diabetes, and dyslipidemia, even in NFAIs raises important questions regarding the pathogenesis and the appropriate management of these until now considered hormonally silent adrenal lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree