Adjuvant Systemic Therapy: Bone-Targeted Treatments
Robert E. Coleman
Bone is the most common site for distant recurrence in breast cancer and is the first location for recurrence in about one-third of patients who relapse. The incidence of bone metastases in metastatic breast cancer is 60% to 80% (1). The development of skeletal metastases involves complex interactions between cancer cells, osteoblasts, and osteoclasts and both hematopoietic and endothelial stem cells within the bone microenvironment. The presence of tumor in bone ultimately results in activation of osteoclasts, leading to an increased rate of bone resorption. Additionally, bone marrow derived stem cells are of fundamental importance in the development of metastases at other sites, preparing the environment for tumor cells to establish a metastasis (2). Drug that are able to target bone, notably the bisphosphonates but also inhibitors of receptor activator of nuclear factor kappa-B ligand (RANKL), a key regulator of bone cell function, provide an additional strategy to prevent metastasis within bone and, potentially, at extraskeletal sites.
Bisphosphonates are potent inhibitors of osteoclastic bone resorption, with proven efficacy in reducing tumorassociated skeletal complications in advanced cancer (3). More recently, clinical studies have investigated the adjuvant use of these drugs in breast cancer, with evaluation of their impact on bone density, metastases, and survival. Additionally, numerous preclinical experiments have shown that the development of bone metastases can be inhibited by either the bisphosphonates or RANKL inhibition, through both bone-mediated and possible direct antitumor mechanisms (4). Synergy between potent aminobisphosphonates such as zoledronic acid with chemotherapy has been demonstrated in mouse models, although the clinical relevance of these observations remains uncertain (5).
The data available to date suggest an increasing role for adjuvant bisphosphonates in the treatment of early-stage breast cancer although, as is discussed below, benefits appear to be confined to the postmenopausal setting (6). A strong need exists for continued clinical and laboratory investigation of these drugs in the adjuvant breast cancer setting.
RATIONALE: THE BIOLOGY OF BONE METASTASES
Healthy bone is in a constant state of remodeling, a process that is essential to preserve the structural integrity of bone and to minimize the risk of fragility fractures. Bone-derived osteoblasts and osteoclasts work together through the influence of cytokines and other humoral fractors to couple formation and resorption. Osteoblasts, derived from fibroblast precursors, produce collagen matrix and contribute to bone formation. Osteoclasts are multinucleated giant cells derived from the macrophage-monocyte lineage, and are the major mediator of bone degradation or resorption. In normal health and bone remodeling, the relationship between osteoblastic bone formation and osteoclastic bone resorption are balanced. However, bone diseases including malignancy disturb this delicate balance and result in a loss of the normal structural integrity of the skeleton.
The process of breast cancer metastasis includes tumor cell seeding, tumor dormancy, and subsequent metastatic growth. The primary tumor releases cells that pass through the extracellular matrix, penetrate the basement membrane of angiolymphatic vessels, and then are transported to distant organs via the circulatory system. Circulating breast cancer cells have a particular affinity for bone. These circulating cells can adhere to the vessels and sinusoids of the bone marrow, invading into the marrow and intertrabecular spaces with the help of adhesion molecules. Tumor cells have been shown to exhibit chemotactic responses to areas of bone undergoing resorption (7). Disseminated tumor cells have been reported in the bone marrow of 30% to 40% of early-stage breast cancer patients at the time of diagnosis (8). Most disseminated tumor cells die, but the bone marrow microenvironment may act as a reservoir for malignant cells and the site for dormant tumor cells that only result in relapse many years after the diagnosis of early breast cancer. Tumor cells have the capacity to produce a wide range of cytokines and growth factors that may increase the production of RANKL and macrophage colony-stimulating factor (M-CSF) from osteoblasts leading to activation of osteoclasts and disturbance of the balance of new bone formation and bone resorption. These
multiple interactions between metastatic tumor cells and the bone microenvironment may contribute to the development of metastases both within and outside bone.
multiple interactions between metastatic tumor cells and the bone microenvironment may contribute to the development of metastases both within and outside bone.
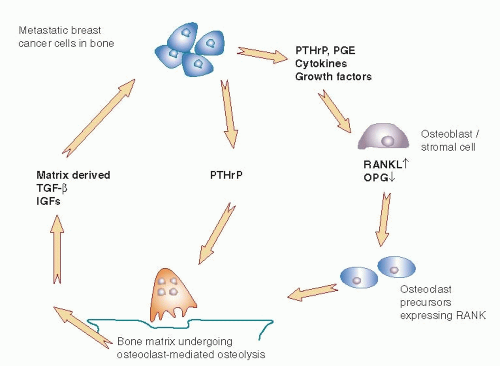
Käkönen and Mundy (9) have described a “vicious cycle” that occurs when cancer cells are present adjacent to the bone matrix (Fig. 47-1). Products produced by the tumor induce breakdown of bone, causing release of factors into the local environment that may cause stimulation and further growth of malignant cells, which in turn leads to yet further bone resorption. At the microscopic level, osteoclasts are visible between cancer cells and the bone surface that is being destroyed (10). Osteoclasts are activated by cytokines produced directly or indirectly by the tumor cell, including parathyroid hormone related peptide (PTHrP), prostaglandins, and interleukins (11). As bone matrix is broken down, a rich supply of mitogenic factors is released, including insulin-like growth factor (IGF-1), platelet-derived growth factor (PDGF), and transforming growth factor β (TGFβ). These factors can lead to increased growth and proliferation of the breast cancer metastases (6, 11). The overall effect is the creation of a self-sustaining vicious cycle with multidirectional interactions between cancer cells, osteoclasts, osteoblasts, and the bone microenvironment.
BISPHOSPHONATES: AGENTS AND MECHANISM OF ACTION
Bisphosphonates are effective in treating conditions in which there is excessive bone resorption and osteoclast activity, including Paget’s disease of bone, osteoporosis, fibrous dysplasia, and hypercalcemia of malignancy. Bisphosphonates have a proven role in reducing skeletal complications in breast cancer patients with bone involvement, and a potential emerging role in the prevention of breast cancer metastases.
Bisphosphonates are analogs of endogenous pyrophosphate, in which a carbon atom replaces the central oxygen atom (Fig. 47-2). As with pyrophosphate, bisphosphonates bind strongly to hydroxyapatite on the bone surface. Unlike pyrophosphate however, which is rapidly split
by the hydrolytic enzymes of osteoclasts, bisphosphonates are stable owing to the central carbon substitution that makes them resistant to hydrolysis. This substitution also allows two additional side chains of variable structure that affect the pharmacologic properties of these agents. One of the side chains usually contains a hydroxyl moiety, which allows high affinity for calcium and bone mineral. The structural variation in the other side chain produces differences in the antiresorptive properties and toxicities (12).
by the hydrolytic enzymes of osteoclasts, bisphosphonates are stable owing to the central carbon substitution that makes them resistant to hydrolysis. This substitution also allows two additional side chains of variable structure that affect the pharmacologic properties of these agents. One of the side chains usually contains a hydroxyl moiety, which allows high affinity for calcium and bone mineral. The structural variation in the other side chain produces differences in the antiresorptive properties and toxicities (12).
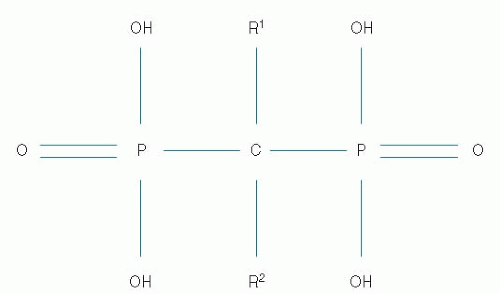 FIGURE 47-2 The basic chemical structure of bisphosphonates includes a central carbon and two variable side chains (R). Variations in the side chains alter the potency and side effects of the drugs. |
Within the family of bisphosphonates, there are more similarities in pharmacologic effects than differences, although side effect profiles, rates of oral absorption, and potency do differ. Although differences in their molecular mechanism of action exist, all therapeutic bisphosphonates have a final inhibitory effect on osteoclast function. Bisphosphonates have a powerful affinity for bone; 40% to 70% of an intravenous administration binds rapidly to the bone surface, preferentially at sites of increased bone formation or resorption with the remainder excreted in the urine. The half-life of bisphosphonates in circulation is less than an hour (13). However, the half-life in bone is very long with biological effects of the most potent agents such as zoledronic acid still evident years after administration of a single dose (14). Once deposited on the bone surface, bisphosphonates are ingested by osteoclasts engaged in bone resorption. These agents interfere with bone resorption by producing a direct toxic apoptotic effect on osteoclasts, and by inhibiting their differentiation and maturation.
Bisphosphonates fall into two classes, based on whether this second side chain is nitrogen-containing or not. Firstgeneration bisphosphonates, such as clodronate and etidronate, do not contain nitrogen. These agents substitute into the production of adenosine triphosphate, which then becomes a toxic adenosine triphosphate analogue that poisons the osteoclast (12). Nitrogen-containing bisphosphonates, such as pamidronate, alendronate, risedronate, ibandronate, and zoledronic acid, interfere with cell signaling and block the prenylation of small signaling proteins that are essential for osteoclast function and survival (12).
Several bisphosphonates are available worldwide for various conditions, with variable antiresorptive potency and route of administration (Table 47-1). The clinical impact of the differences in relative potency between bisphosphonates is not well documented, because only a few direct clinical trial comparisons have been conducted. Although all bisphosphonates can theoretically be administered either orally or intravenously, the oral bioavailability of any bisphosphonate is extremely limited and so for some only intravenous formulations have been developed. The dose and frequency of administration varies depending on the clinical indication, with doses used to treat bone metastases being about 10-fold higher than those used to treat osteoporosis. For example, the zoledronic acid dose approved for treating bone metastases is 4 mg intravenously every 3 to 4 weeks. When used in the treatment of osteoporosis, it is approved as a 5-mg dose once yearly. Ibandronate, available in both oral and intravenous forms, is given 50 mg orally daily or 6 mg intravenously monthly for bone metastases, compared with 150 mg orally monthly or 3 mg intravenously every 3 months for osteoporosis. In the United States, ibandronate is approved only for the osteoporosis indication. Doses of bisphosphonates under investigation in the adjuvant breast cancer setting for prevention of metastases have ranged from full bone metastasis treatment doses to somewhat lower doses or less frequent administrations more similar to the osteoporosis treatment schedules.
TABLE 47-1 Bisphosphonates: Antiresorptive Potency and Route of Administration | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
BISPHOSPHONATES AS ADJUVANT THERAPY IN EARLY-STAGE BREAST CANCER
Preclinical data have provided biologic plausibility for a role of bisphosphonates in inhibiting the development of bone metastases. In vitro studies have demonstrated that bisphosphonates can inhibit critical steps in development of metastases in the bone, including adhesion and invasion (15). Animal tumor model systems have shown that bisphosphonates can inhibit development of bone metastases, reduce tumor burden in the bones, and improve survival in nude mice injected with human breast cancer cells (5, 16, 17). Although most animal models suggest that the primary antitumor effect of bisphosphonates is manifested in the bone, some data indicate also an effect of bisphosphonates on extraskeletal metastases. The potential mechanisms involved are summarized in Figure 47-3A. Laboratory experiments have shown also that bisphosphonates can have an impact on cancer cells through antiangiogenic, anti-invasive, and immunomodulatory mechanisms (18, 19 and 20). Additionally, nitrogen-containing bisphosphonates can directly induce tumor cell apoptosis, inhibit tumor cell proliferation, and synergistically with cytotoxic chemotherapy agents commonly used in breast cancer treatment (5, 16, 21). The high doses of bisphosphonates that have been used in many laboratory studies suggesting direct anticancer activities are incompatible with the clinical doses and schedules approved for the treatment of cancer patients. Whether a direct antitumor effect of bisphosphonates plays a clinically significant role in the treatment or prevention of cancer in humans remains unproved.
Although bone-targeted treatments have had a profound effect on skeletal morbidity and quality of life in advanced breast cancer, clear improvements in survival of advanced cancer patients have not been seen. The studies may have been underpowered to detect survival advantages or, more likely, reflect the futility of modifying the bone microenvironment to influence the large burden of disease present with overt metastases. However, in the adjuvant setting, where the disease burden is microscopic and potentially more receptive to cellular changes in the bone marrow, the anticancer effects of bone-targeted treatments seen in the preclinical models are more likely to be mirrored in patients.
Phase II exploratory studies in women with earlystage, high-risk breast cancer have reported that monthly
zoledronic acid, in combination with standard anticancer therapy, was able to increase the clearance of disseminated tumor cells (DTC) and reduce the number and persistence of DTC in bone marrow compared with standard therapy alone (22, 23 and 24). The zoledronic acid-mediated reduction in DTC persistence might be one of the mechanisms underlying the observed clinical benefits in the studies described below. However, further studies are needed to determine whether the disease-modifying benefits seen in some patient subsets correlate with decreases in DTC levels.
zoledronic acid, in combination with standard anticancer therapy, was able to increase the clearance of disseminated tumor cells (DTC) and reduce the number and persistence of DTC in bone marrow compared with standard therapy alone (22, 23 and 24). The zoledronic acid-mediated reduction in DTC persistence might be one of the mechanisms underlying the observed clinical benefits in the studies described below. However, further studies are needed to determine whether the disease-modifying benefits seen in some patient subsets correlate with decreases in DTC levels.
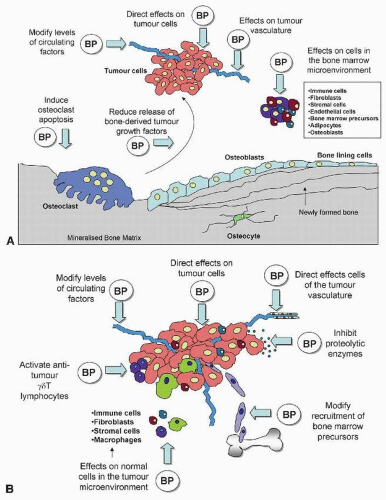 FIGURE 47-3 Potential antitumor effects of bisphosphonates within (A) and outside (B) bone. BP, bisphosphonates. |
The early adjuvant clinical trials evaluated oral bisphosphonates with three using daily oral clodronate (25, 26 and 27) and a fourth daily oral pamidronate (28). Clodronate is approved for the treatment of metastatic bone disease in many parts of the world (except the United States) while the development of oral pamidronate was abandoned due to poor absorption and gastrointestinal toxicity at the high doses required for effects on bone metabolism.
The adjuvant trials evaluating oral clodronate provided promising but conflicting results (25, 26 and 27). In the first study, 302 women with breast cancer and DTC detected using immunocytochemistry in a bone marrow aspirate (but no overt metastases) taken at the time of diagnosis were randomized to receive either clodronate (1,600 mg/day) or no bisphosphonate for 2 years. Additionally, patients received standard adjuvant systemic therapy (25). Patients who received clodronate had a lower incidence of bone metastases (p = .003), and a significantly longer bone metastasis-free survival (p < .001). A final analysis at 8.5 years of follow-up continued to show a significant improvement in overall survival for patients given clodronate, although the significance in disease-free survival (DFS) no longer persisted (29).
A larger, randomized, placebo-controlled, multinational trial was conducted in which 1,079 patients with early-stage breast cancer were randomized to receive either clodronate (1,600 mg/day) or placebo for 2 years in addition to standard systemic therapy (26). Patients were assessed for bone metastases at 2 and 5 years, and as clinically indicated. The final analysis showed that oral clodronate significantly reduced the risk of bone metastases at 2 years (hazard ratio [HR] 0.546; p = .03) and 5 years (HR 0.692; p = .04). A significant reduction was also seen in mortality (HR 0.768, p = .048). Follow-up showed a continued separation of the survival curves between years 5 and 10 (26). This reduction
was largely restricted to patients who were postmenopausal at diagnosis of breast cancer.
was largely restricted to patients who were postmenopausal at diagnosis of breast cancer.
The third study of oral clodronate was a randomized, open-label study investigating 3 years of adjuvant clodronate therapy (1,600 mg/day) in 299 patients with lymph node-positive breast cancer (27). This study showed no reduction in bone metastases in the clodronate treated arm, and, at least at the time of the initial report, a worrisome increase in visceral metastases and a reduction in overall survival at 5 years for patients receiving clodronate. At the final analysis after 10 years, survival was similar in both groups (30). This small study also had some imbalances in important prognostic factors that may explain the apparent adverse effects with clodronate. Nevertheless, this study had a disproportionate effect on the perception of adjuvant clodronate as an adjuvant treatment and regulatory approval based on the other two positive studies was not achieved. The Danish study with oral pamidronate also failed to show any significant effect on disease outcomes, perhaps due to the poor absorption and patient adherence to treatment (28).
It was not until the first published results of the Austrian Breast and Colorectal Cancer Study Group (ABCSG) 12 trial in 2009 (31) that the potential role of adjuvant bisphosphonates was really appreciated by the breast cancer community and the old “seed and soil” theory of metastasis described in the 19th century by Sir Stephen Paget (32) regained influence in the search for further improvements in the outcome of patients with early breast cancer. The ABCSG 12 trial enrolled 1,803 premenopausal women with estrogen-receptor-(ER-) positive breast cancer. All patients received ovarian suppression for 3 years with goserelin. Patients were randomized in a 2 × 2 design to receive tamoxifen versus anastrozole, and zoledronic acid (4 mg every 6 months) or not.
At the first efficacy analysis, reported after 137 events (70 distant relapses) with approximately 60 months of follow-up, no difference was seen in outcome with respect to the endocrine therapy randomization. There was, however, a statistically significant improvement in DFS for the patients who received zoledronic acid (HR 0.64; p = .01). Patients who received zoledronic acid had fewer recurrences at all sites including visceral metastases. Additionally, locoregional recurrence was also less frequent with zoledronic acid. These results were maintained at a later analysis after a median follow-up of 62 months (33). Moreover, after further follow-up (median = 84 months) a persistent benefit in DFS more than 3 years after completion of treatment (HR = 0.71, p = .011) suggested a sustained “carryover” benefit from adding zoledronic acid to endocrine therapy (34). In addition at this most recent analysis, there were sufficient deaths in this generally good prognosis population of patients to show that treatment with zoledronic acid also significantly improved overall survival (OS) compared with the control group (HR = 0.61, p = .033). Interestingly, and of relevance when the results of the other adjuvant studies are discussed later, this most recent analysis of ABCSG-12 showed that the benefits of zoledronic acid appeared to be confined to women over the age of 40 at diagnosis in whom ovarian suppression with goserelin can be expected to reliably fully suppress estrogen and other reproductive hormone production. In women aged 40 years or younger (n = 413), no statistically significant difference in DFS was observed between the zoledronic acid-treated and control groups (HR = 0.87, p = .53). However, among women aged 40 years or more at study entry (n = 1,390), zoledronic acid resulted in a 34% reduction in the risk of DFS events compared with the control group of patients (HR = 0.66, p = .013). Zoledronic acid was also associated with a statistically significant improvement in OS in this older subset of patients (HR = 0.57, p = .042) (34).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree