Study
Patient population
Treatment
Results
NATO (N = 605)
[64]
Nodal positive, premenopausal; nodal positive or negative, postmenopausal
T × 2 years (N = 300)
vs.
No treatment (N = 305)
Better PFS and OS with T
NSABP B-14
(N = 2644)
[65]
Nodal negative,
≤49 years and ≥50 years
T × 5 years
vs.
Placebo
T > placebo DFS 83% vs. 77%;
Tam decreased RR 44%;
no OS difference
NSABP B-14
Tam > 5 years
(N = 1172)
[66]
Nodal negative
≤49 years and ≥50 years
(after 5 years T)
T × 5 more years (N = 593)
vs.
Placebo (N = 579)
No DFS/OS difference at 4 years;
T < placebo at 7 years
Scottish trial
(N = 1323)
[67]
Nodal negative, premenopausal and postmenopausal
T × 5 years adjuvant (N = 667)
vs.
T after relapse (N = 656)
T > no treatment
DFS/RFS
No impact on OS
Scottish trial
Tam beyond 5 years
(N = 342)
Nodal negative (after 5 years T)
T (N = 173)
vs.
No further therapy (N = 169)
No difference except increase in endometrial cancer with T > 5 years
GROCTA trial
(N = 504)
[70]
Nodal positive, premenopausal (N = 237)
T × 5 years
vs.
CMF × 6, E × 4
vs.
CMF × 6, E × 4, T x 5 years
No difference in DFS/OS between Tam and chemo in premenopausal women, except an excess of locoregional recurrences with T
GABG study
(N = 331)
[71]
Nodal positive
< 50 years
CMF × 6
vs.
T × 5 years
CMF > T for DFS/OS
ECOG study
(N = 533)
[72]
Nodal positive
ER positive and ER negative
Chemotherapy
vs.
chemotherapy, T X 5–10 years
T > no treatment after chemotherapy
IBCSG 13–93
(N = 1246)
[17]
Nodal positive, pre- and perimenopausal
AC × 4, CMF × 3, T × 5 year
vs.
AC × 4, CMF × 3, no treatment
T > no treatment for DFS
Side effects of tamoxifen
Tamoxifen treatment in premenopausal women is associated with a variety of symptoms, including vasomotor symptoms, vaginal complaints (dryness, itching and discharge), decrease of libido, amenorrhea, insomnia and mood disturbances, leading to significant restriction in the quality of life [15]. The small absolute increase in the incidence of thromboembolic events and uterine cancer are both seen mostly in women older than 55 years.
The role of AIs in combination with OFS has been assessed in the ABCSG12 trial as well as in the SOFT and TEXT trials. The ABCSG12 trial was a randomized, controlled, open-label, two-by-two factorial, multi-centre trial. It recruited 1803 premenopausal women with endocrine-sensitive early breast cancer receiving the LHRH antagonist (goserelin) and compared the efficacy and safety of anastrozole and tamoxifen with or without zoledronic acid for 3 years [20]. At a median follow-up of 62 months (range 0–114.4 months), overall no difference was detected in disease-free survival between women receiving A or T (HR 1.08, 95% CI 0.81–1.44; p = 0.591), but those on anastrozole had inferior overall survival (46 vs. 27 deaths; HR 1.75, 95% CI 1.08–2.83; p = 0.02). The addition of zoledronic acid improved disease-free survival (HR 0.68, 95% CI 0.51–0.91; p = 0.009).
The recently reported Suppression of Ovarian Function (SOFT) trial was designed to evaluate whether OFS in combination with either tamoxifen or an AI (exemestane) added benefit compared to tamoxifen alone in premenopausal women with hormone-sensitive early breast cancer receiving endocrine treatment alone or who remained premenopausal after adjuvant chemotherapy. In women, who remained premenopausal after adjuvant chemotherapy, adding OFS to tamoxifen resulted in an absolute increase in 5-year breast cancer-free survival of 4.5%. The benefit was even greater when comparing tamoxifen alone versus exemestane plus OFS, with a 5-year absolute increase in breast cancer-free and distant recurrence-free survival of 7.7% and 4.2%, respectively [21]. No OS data have been provided so far.
The TEXT trial randomized premenopausal women receiving OFS to exemestane or tamoxifen. In contrast to the ABCSG12 trial, women randomized to the AI and OFS arm had significantly better disease-free survival compared to the tamoxifen and OFS arm (HR, 0.72). Overall survival between the groups was not significantly different (HR, 1.14) [22].
Based on these results, an expert panel of the American Society of Clinical Oncology (ASCO) recently published an update of the clinical guidelines on the use of OFS in premenopausal women with hormone-sensitive early breast cancer [23]. These guidelines are in line with the recommendations of the St. Gallen Consensus Conference 2015 [24]:
The Panel recommends that higher-risk patients should receive ovarian suppression in addition to adjuvant endocrine therapy, whereas lower-risk patients should not.
Women with stage II or stage III breast cancers who would ordinarily be advised to receive adjuvant chemotherapy should receive ovarian suppression in addition to endocrine therapy.
Women with stage I or II breast cancers at higher risk of recurrence, who might consider chemotherapy, may also be offered ovarian suppression in addition to endocrine therapy.
Women with stage I breast cancers not warranting chemotherapy should receive endocrine therapy but not receive ovarian suppression.
Women with node-negative cancers 1 cm or less (T1a, T1b) should receive endocrine therapy but not receive ovarian suppression (◘ Table 35.2).
Table 35.2
Ovarian suppression studies
Study | Patient population | Treatment | Results |
|---|---|---|---|
Ovarian ablation vs. chemotherapy | |||
Scottish trial (N = 332) [73] | Nodal positive | CMF × 6–8 cycles vs. Goserelin x 2 years | No difference in event-free or OS Goserelin better in HR+ CMF better in HR- |
Scandinavian study (N = 732) [74] | Nodal positive or T > 5 cm | CMF × 9 cycles vs. Ovarian irradiation | No difference |
TABLE study (N = 589) [75] | Nodal positive | CMF × 6 cycles vs. Leuprorelin x 2 years | No difference |
ZEBRA study (N = 1640) [76] | Nodal positive | CMF × 6 cycles vs. Goserelin × 2 years | No difference for HR+ CMF better for HR- |
Ovarian ablation + chemotherapy | |||
INT0101 study (N = 1503) [77] | Nodal negative | CAF × 6 vs. CAF × 6 + G x 5 years vs. CAF × 6 + GT x 5 years | Better TTR/DFS with CAFGT No diff OS CAFG better for <40 yrs.? |
ZIPP trial (N = 2648) 43% of enrolled patients also received chemotherapy [78] | Stage I/II | T × 2 years vs. G × 2 years vs. TG × 2 years vs. No hormonal treatment | G better for DFS/OS |
IBCSG 11–93 (N = 174) [79] | Nodal positive | AC × 4 + OA/OS + T × 5 years vs. OA/OS + T × 5 years | No difference |
IBCSG VIII (N = 1063) [80] | Stage I/II, nodal negative | G × 2 years vs. CMF × 6 vs. CMF × 6, Z × 1.5 years | CMF > G in HR- patients CMF = G in ER+ patients |
Arriagada et al. (N = 926) [81] | Nodal positive, high grade 63% HR+ 77% received anthracycline-based chemo | Chemo vs. chemo + OA/OFS | No difference overall Lower recurrence <40, ER+ |
Ovarian ablation + tamoxifen | |||
INT0142 (N = 350) closed early due to poor accrual [82] | Nodal negative | T × 5 years vs. OA + T × 5 years | No difference |
Vietnamese (N = 709) [83] | Stage I/II | OA + T vs. observation | Better DFS/OS with OA + T |
GROCTA (N = 244) [84] | Nodal positive | G + T vs. CMF × 6 | No difference |
FASG06 (N = 333) [85] | Nodal positive | Triptorelin x 3 years + T vs. FEC × 6 | No difference |
French (N = 162) [86] | Nodal positive | OA + T vs. FAC | No difference |
ABC (OAS) (N = 2144) [13] | Stage I/II/III | T ± chemo + OA/OFS vs. T ± chemo | No difference |
ABCSG5 (N = 1045) [87] | Stage I/II | G x 3 years + T × 5 years vs. CMF × 6 | Better DFS with G + T No difference in OS |
Ovarian ablation + tamoxifen vs. AIs | |||
ABCSG12 | Premenopausal Stage I/II/III | G + A vs. G + A + Z vs. G + T vs. G + T + Z | No difference in DSF |
IBSCG 24–02/BIG 2–02: SOFT [21] | Premenopausal No adjuvant chemotherapy or premenopausal after adjuvant chemotherapy | T vs. OFS + T vs. OFS + E | No difference in DSF |
IBSCG 25–02/BIG 3–02: TEXT [22] | Premenopausal women with HR+ tumours who require OS with or without chemotherapy from the start of adjuvant therapy | OFS + T vs. OFS + E | In the combined analysis SOFT/TEXT: DSF better for OFS + E |
Side effects of OFS
The main side effects of OFS are due to estrogen deprivation leading to premature menopause and include hot flashes, sweats, weight gain and decreased libido as well as sleep disturbances, mood alterations and bone loss. In the Nurse’s Health Study, bilateral oophorectomy was associated with a significant increase in all-cause mortality [25], but this effect was seen only in women who underwent oophorectomy due to benign disease. The risk of cardiovascular disease and stroke may also be increased after OFS: A meta-analysis of 18 studies found a significantly increased risk of cardiovascular disease in women who underwent bilateral oophorectomy compared with premenopausal women of the same age (RR 2.62, 95% CI, 2.05–3.35) [26]. The addition of tamoxifen or an AI to OFS may intensify postmenopausal symptoms and musculoskeletal side effects as reported in the quality of life (QoL) sub-studies of the SOFT and TEXT trials, but differences in symptom-specific QoL were less pronounced for patients with prior chemotherapy [27, 28]. Furthermore, the addition of OFS to oral adjuvant endocrine therapy seems not to affect cognitive function in a clinically meaningful way after one year of treatment [29].
35.4 Endocrine Treatment in Postmenopausal Women
Due to cessation of hormone production by the ovaries, the level of circulating estrogen after the menopause is substantially lower than in the premenopausal women. For therapeutic purposes, the effect of estrogen on hormone-sensitive breast cancer cells can be additionally reduced with selective estrogen receptor modulators (SERMs) like tamoxifen [30] or with selective estrogen receptor downregulators (SERDs) like fulvestrant [31]. Alternatively the level of circulating estrogen can be lowered with the use of aromatase inhibitors (AIs) [32]. These drugs block the extragonadal transformation of androgen to estrogen, in particular in the adrenal glands and in several other tissues. In postmenopausal women, this aromatization is the primary source of estrogen.
Tamoxifen is a SERM with a predominantly antagonistic effect on the ERs in breast tissue, a partial agonistic effect in bone, the cardiovascular system and the CNS and an agonistic effect on the uterus, liver and vaginal mucosa. For more than 30 years, tamoxifen has been the drug of choice in the treatment of hormone-sensitive breast cancer, leading to a reduction in disease recurrence and in contralateral breast cancer of about 50% and of mortality by around 30% [12, 16]. In early breast cancer, treatment with tamoxifen for 5 years reduces the recurrence rate by about half during treatment and by about one-third in the subsequent 5 years and reduces breast cancer mortality by almost one-third throughout the first 15 years [15]. Extending tamoxifen treatment to 10 years induces a further mortality reduction during years 10 to 14 [18, 33] by about 20%.
In the concomitant administration of tamoxifen and chemotherapy in postmenopausal women, tamoxifen seems to diminish the efficacy of anthracycline-based chemotherapy [34, 35], and it should be avoided. Few or no data are available for the interaction of tamoxifen with other treatments like taxanes, trastuzumab or radiotherapy [36].
The conversion of tamoxifen to the active metabolite endoxifen is dependent on the activity of cytochrome P450 2D6 (CYP2D6). During the last decades, several studies have reported on the potential association between CYP2D6 polymorphism and tamoxifen treatment outcomes, with highly inconsistent results [37, 38]. The recently published ASCO Guidelines about the use of biomarkers to guide decisions on adjuvant systemic therapy couldn’t support the use of CYP2D6 polymorphism to decide which patients may benefit from tamoxifen [39]. Association between vasomotor or musculoskeletal symptoms, metabolism and outcome has been extensively reported. Poor or intermediate metabolism phenotypes seem to be associated with tamoxifen-induced hot flushes but not with inferior outcomes in term of disease-free survival [40]. On the other hand, patients reporting arthralgia/myalgia symptoms may have favourable disease-free survival and breast cancer-free interval [41].
Side effects of tamoxifen
Thromboembolic events, in particular deep vein thrombosis and pulmonary embolism, as well as the increased incidence of cerebrovascular accidents represent the more severe side effects of tamoxifen. Tamoxifen use can also lead to hot flushes, vaginal discharge and sexual dysfunction [42]. An increased risk of endometrial cancer (◘ Fig. 35.1) seems to be more frequent in women over 55 years of age [15]. Recent data reported cognitive function disturbance at least in the short term after treatment initiation [43], with women showing greater degrees of reduction of psychomotor and motor speed in patients treated with AIs compared with patients taking tamoxifen.
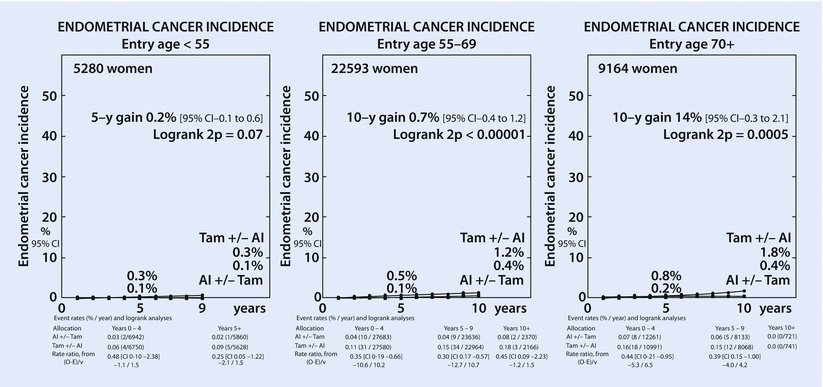

Fig. 35.1
Endometrial cancer incidence (Reprinted from Ref. [44], Copyright © Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Open-access article distributed under the terms of the Creative Commons Attribution License CC BY. ► http://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(15)61074-1.pdf)
A recently published meta-analysis using individual data from almost 32000 postmenopausal women with hormone-sensitive early breast cancer participating in clinical trials showed that 5 years of aromatase inhibitor treatment reduces the relative recurrence rates by about 30% compared with 5 years of tamoxifen during the treatment phase, but not thereafter. Five years of an aromatase inhibitor reduces 10-year breast cancer mortality rates by about 15% if compared with 5 years of tamoxifen and by about 40% (proportionately) if compared with no endocrine treatment at all [44]. In particular in trials comparing 5 years of tamoxifen versus AI treatment (n = 9885), AIs reduced breast cancer recurrence during years 0 to 1 (RR 0.64, 95% CI 0.52–0.78) and years 2 to 4 (RR 0.80, 95% CI 0.68–0.93) and lowered breast cancer mortality (RR 0.85, 95% CI 0.75–0.96). Five years of AI also reduce the incidence of contralateral breast by about 1.5% in ten years if compared with 5 years of tamoxifen (incidence rate of 2.1% for AI versus 4.7% for tamoxifen). In trials comparing a five-year course of tamoxifen versus 2–3 years of tamoxifen followed by an AI to complete 5 years of endocrine treatment (n = 11,798), the switch to an AI resulted in a reduced breast cancer recurrence rate during years 2–4 (RR 0.56, 95% CI 0.46–0.67) and fewer deaths from breast cancer (RR 0.84, 95% CI 0.72–0.96) as well as an absolute reduction of contralateral breast cancer incidence by 0.8%. Finally, in trials, which compared 5 years of AI alone versus 2–3 years of tamoxifen followed by an AI, the treatment with an AI alone resulted in a lower recurrence rate during years 0 to 1 (RR 0.74, 95% CI 0.62–0.89) and similar recurrence rates in years 2 to 4 (RR 0.99, 95% CI 0.85–1.15) as well as a trend towards reduced breast cancer mortality (RR 0.89, 95% CI 0.78–1.03) but no relevant reduction in contralateral breast cancer (0.3%) .
In the same meta-analysis, the sequence of AI followed by tamoxifen as investigated in the BIG 1–98 trial [45–47] was not included. In the BIG 1–98 trial, 8000 women were randomized to receive 5 years of tamoxifen or letrozole or a sequential treatment with 2.5 years of tamoxifen followed by 2.5 years of letrozole or vice versa. Monotherapy with letrozole showed a better outcome compared with tamoxifen alone, but there was no significant difference between sequential therapies with only 2.5 years of AI and letrozole alone for 5 years.
Recently data about extended endocrine treatment with an AI has been published. A total of 1918 patients were randomly assigned to receive letrozole (959 patients) or placebo (959 patients) after 4.5 to 6 years of AI [72]. The 5-year disease-free survival rate was 95% (95% confidence interval [CI], 93 to 96) with letrozole and 91% (95% CI; 89 to 93) with placebo [48] (◘ Table 35.3).
Table 35.3
Tamoxifen vs. Aromatase Inhibitor Studies
Study | Patient population | Treatment | Results |
|---|---|---|---|
ATAC (N = 9366) | ER positive or unknown | Tamoxifen versus anastrozole versus combination for 5 years | Anastrozole better PFS than tamoxifen or combination |
BIG1–98 (N = 8010)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




