- Lack of insulin is the culprit in diabetic ketoacidosis.
- The most common precipitating cause is infection.
- The classic signs include polyuria and polydipsia, rapid weight loss, weakness, Kussmaul respiration, drowsiness and, rarely, coma.
- The cornerstones of treatment are rehydration, IV insulin and potassium supplementation.
- Intravenous insulin administration should continue until the acidosis is normalized (i.e. not merely until euglycemia is achieved).
- This is characterized by hyperosmolality with progressive hyperglycemia of >35—40 mmol/L and an effective serum osmolality of >320mOsm/kg.
- It typically affects elderly patients with type 2 diabetes.
- The most common precipitating causes are infection and cardiovascular disease.
- Treatment involves aggressive rehydration and IV insulin.
Introduction
Both diabetic ketoacidosis (DKA) and hyperosmolar non-ketotic hyperglycemia (HH) are caused by a lack of insulin leading to unrestricted flux of stored lipid, carbohydrate and amino acid nutrients into the blood. These conditions are both acute and life-threatening, and represent the ultimate metabolic consequences of deranged type 1 (T1DM) and type 2 diabetes mellitus (T2DM), respectively [1–3]. The hallmark of DKA is a high-anion-gap metabolic acidosis caused by a rapidly progressive excess of ketoacids (3-hydroxybutyrate and acetoacetate – “ketone bodies”) while severe hyperosmolarity caused by hyperglycemia is the most notable feature of HH. The distinction is not always obvious on clinical grounds; patients with DKA may be very hyperosmolar and ketone body levels are in general somewhat elevated in HH. Although the clinical picture may vary considerably depending on co-morbidities, differential diagnosis seldom poses any major problem and in the rare cases in which distinction remains difficult, treatment generally follows the same principles, regardless of whether ketosis or hyperglycemia is the most urgent clinical challenge.
Mortality rates have been steadily declining over the past few years [4], but remain close to 5% for DKA and 10–15% for HH [2,5]. The decline in mortality may be a consequence of lower incidence of DKA and HH, earlier diagnosis, improved treatment or, more plausibly, all of these effects combined. Improved education schedules and self-monitoring (e.g. blood ketone testing), organization of specialized diabetes clinics and the use of standardized low-dose insulin regimens have also contributed to this favorable trend [2,6].
Diabetic ketoacidosis
Definitions
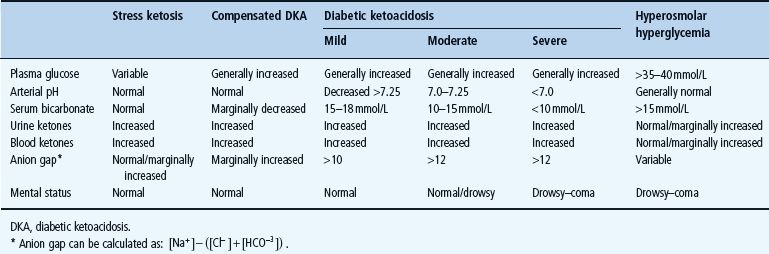
Diabetic ketoacidosis is the most common, serious and demanding medical emergency within the fields of diabetology and endocrinology. There is no generally accepted definition of DKA and, in particular, very mild cases may be difficult to diagnose. At a minimum, it is reasonable to require that pH is below the normal range and that the levels of ketoacids (ketone bodies) in the blood or urine are markedly elevated. As outlined in Table 34.1, there is a continuous deterioration from clinically insignificant “stress” ketosis to full-blown severe ketoacidosis. In the US population, it has been estimated that 2–8% of hospital admissions in children with diabetes are a result of DKA and that the annual incidence rate of DKA in children is around 5 per 1000 patients [1].
Table 34.1 Classification of clinical pictures and diagnostic criteria. Adapted from Standards of Medical Care in Diabetes — 2004/2006 position statement. Diabetes Care 2004; 27:S94—S101, with permission from the American Diabetes Association.
Pathogenesis and pathophysiology
Insulin deficiency
DKA is caused by insulin deficiency. Insulin deficiency may be relative (e.g. in the setting of severe infection) where normal amounts of insulin are insufficient, or absolute when insulin therapy is neglected. Lack of insulin leads to uncontrolled lipoly-sis and ketogenesis and increases plasma glucose. Although often disregarded it should also be borne in mind that insulin deficiency, most particularly when longstanding, causes increased breakdown of body protein, as evidenced by the extreme sarco-penia and cachexia of patients with T1DM prior to the insulin era. Excessive protein breakdown and ensuing release of ketogenic and gluconeogenic amino acids may contribute to ketosis and hyperglycemia.
Stress hormones and cytokines
At some stage insulin deficiency becomes coupled with excess of “counter-regulatory” or “stress” hormones and cytokines [7,8]. Release of stress hormones may in part be triggered by cytokines and in part by general stress, such as dehydration, hypotension and hypoperfusion. It has been shown that both endotoxin and tumor necrosis factor (TNF) mimics all metabolic responses to infection including hyperthermia and stress hormone release [9,10]. The traditional stress hormones include glucagon, epinephrine, growth hormone (GH) and cortisol, all of which have well-described metabolic actions. Glucagon and epinephrine have a rapid onset of action, whereas GH and cortisol act with a latency of hours.
Cytokine levels are elevated in DKA, even in the absence of infection. The metabolic actions of cytokines are in general not so well understood and it is possible that many of these actions are mediated by hypothalamo-pituitary activation and subsequent stress hormone release. Certain cytokines, such as TNF-α, may impair insulin sensitivity in peripheral tissues [11,12] and a vicious circle may thus be initiated with self-perpetual increments in blood glucose and cytokine levels. Insulin has anti-inflammatory properties in critically ill patients [13], and administration of exogenous insulin may in itself increase insulin sensitivity [14], conceivably to some extent by breaking this vicious circle but also because glucotoxicity and lipotoxicity wane.
Lipid metabolism
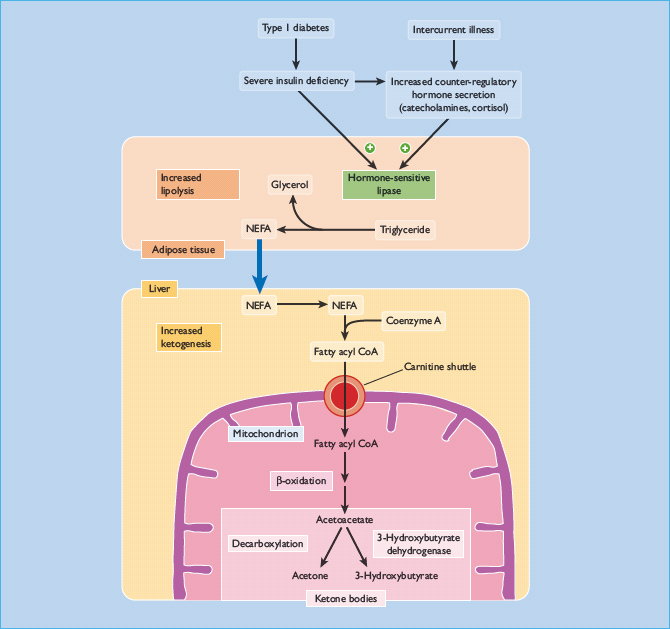
Contrary to popular belief, deranged lipid – not carbohydrate – metabolism is the main cause of DKA. In essence, DKA is brought about by uncontrolled lipolysis in adipose tissue and uncontrolled ketogenesis in liver.
Adipose tissue is present in regional depots such as subcutaneous upper and lower body and visceral fat [15]. Apart from these classic depots, fat is present in most other tissues (e.g. connective tissue, bone marrow, liver and muscle). The picture is further complicated by the fact that within each tissue fat is distributed in compartments. In muscle, for instance, fat is present intramyo-cellularly, intermyocellularly and intermuscularly. Under physiologic conditions, lipolysis is tightly controlled by lipases. Hormone-sensitive lipase and probably also adipose triglyceride lipase stimulate release of free fatty acids and glycerol into the circulation. This process is inhibited by insulin and low insulin levels increase lipolysis swiftly. The stress hormones, such as epinephrine, growth hormone and cortisol, stimulate lipolysis. It is plausible that dehydration per se also participates in the stimulation of lipolysis [16]. These events take place in the course of hours and may rapidly triple or quadruple blood concentrations of free fatty acids.
Ketogenesis occurs in the liver by oxidation of free fatty acids to ketoacids or ketone bodies (Figure 34.1). Ketone bodies, in particular 3-OH-butyrate, are phylogenetically ancient fuel compounds, which are present and prominent in very primitive species [17], suggesting that they have had an important role throughout evolution for the past 2–3 billion years. Physiologically, ketone bodies provide important fuel energy for the brain and other tissues under fasting, prolonged exercise and other conditions of fuel shortage. In DKA, ketogenesis becomes uncontrolled and circulating levels of ketone bodies rise rapidly and excessively. This occurs because of both increased supply of fatty acids to the liver and because low levels of insulin and high levels of glucagon in the liver promotes ketogenesis [18]. In normal individuals this unrestrained process is prevented by compensatory rises in insulin secretion, but this does not occur in those with T1DM.
Glucose metabolism
Hyperglycemia is usually present in DKA, but it is important to stress that DKA not infrequently presents with normal or modestly elevated glucose concentrations [19]. This may particularly be the case during caloric deprivation caused by gastrointestinal disease, for example. Hyperglycemia is caused by a combination of lack of insulin and an excess of stress hormones, leading to insulin resistance. In the liver this increases gluconeogenesis and hepatic glucose production. The kidney is unlikely to have any significant role in the initial stages of DKA [20]. The ensuing high glucose levels generate a high flux state with increased peripheral glucose disposal, but the increased mass action of glucose is generally insufficient to compensate fully. Muscle glucose metabolism is characterized by insulin resistance because of high levels of stress hormones, high levels of free fatty acids and varying degrees of dehydration.
Precipitating factors
In patients with known diabetes, DKA is usually precipitated by a coexisting illness or by omission of insulin therapy. The most common factor is infection, ranging from trivial viral infections to full-blown septicemia. Other precipitating factors include cardiovascular events (myocardial infarction, stroke), gastrointestinal disease, inflammatory diseases, pancreatitis, trauma and major surgery, alcohol abuse and drugs, especially glucocorti-coids. All of these factors induce insulin resistance because of the stress hormone responses. Furthermore, poor appetite and food deprivation will often lead the patient to take less insulin. In this context, gastrointestinal disease with nausea and vomiting pose a specific problem and it may be necessary to admit such patients to the hospital for intravenous glucose and insulin therapy. Diabetes ketoacidosis may be a presenting feature of new onset T1DM (see Chapter 19).
Psychologic factors also play an important part. Poor compliance is commonly seen in younger patients, patients with psychiatric illnesses and in minority groups who unfortunately may have a poor understanding of diabetes care principles for linguistic or cultural reasons.
Diagnosis and clinical presentation
DKA usually develops over a short period of time, generally in less than 24 hours. There may have been some antecedent days with general malaise and poor metabolic control. Depending on the degree of hyperglycemia, the history will include symptoms of polydipsia and polyuria (Table 34.2). Specific symptoms depend on the precipitating factors and other co-morbidities that might be present. Physical examination may reveal poor skin turgor, hyperventilation (Kussmaul respirations), hypotension, tachycardia and impairment of mental state. Many patients have infections but with normothermia or even hypothermia, caused by peripheral vasodilatation brought about by the acidemia.
Table 34.2 Common clinical features of diabetic ketoacidosis.
| Polyuria, polydipsia Rapid weight loss Muscular weakness Visual disturbance Air hunger with Kussmaul respiration, dry lips Abdominal pain, leg cramps Nausea, vomiting Confusion, drowsiness, coma |
Prompt diagnosis and initial treatment rests on:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree