Assessing Risk in those with NSTEACS
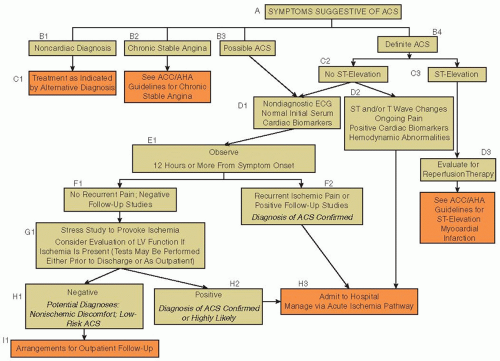
After the initial triage, patients presenting with ACS without ST-segment elevation (non-ST-elevation ACS [NSTEACS]) or hemodynamic compromise can be evaluated in a more systematic manner, with the intent of determining the probability that the patient’s symptoms are due to coronary artery disease (CAD). This involves a careful review of the patient’s symptoms and physical findings, analyzing the ECG for ischemic changes such as ST-segment depression or T-wave inversion, reviewing the patient’s medical record for a personal history of CAD or risk factors for heart disease, and evaluating serial serum biomarkers such as troponin to assess for MI.
The initial history is critical to assessing the probability of ACS. Typical angina is usually described as a deep substernal chest discomfort that can radiate to the arm and jaw and is associated with shortness of breath or diaphoresis. In ACS, these symptoms may present with rest pain or accelerating angina that occurs with lessening degrees of activity. For older patients, women, and diabetics, these typical features may not be present and are unreliable. Despite the widely held belief to the contrary, the relief of chest pain by sublingual nitroglycerin does not predict the presence of ACS.
43The ECG provides important prognostic and diagnostic information at the time of initial triage. It is an essential component of the early triage algorithm. Findings of old Q waves on the ECG are important because they indicate likely prior MI and a higher risk for CAD. A normal ECG does not exclude the diagnosis of ACS, but patients who have an unchanged ECG when compared to a prior one have a low risk of adverse events.
44
Cardiac Biomarkers
The evaluation of cardiac biomarkers is an essential component to early triage and risk stratification. The clinical diagnosis of MI hinges on the detectable elevation of these cardiac biomarkers in conjunction with other findings of ischemia such as symptoms or ECG changes. Biomarkers are released into the peripheral circulation from infarcted cardiac myocytes when necrosis occurs (
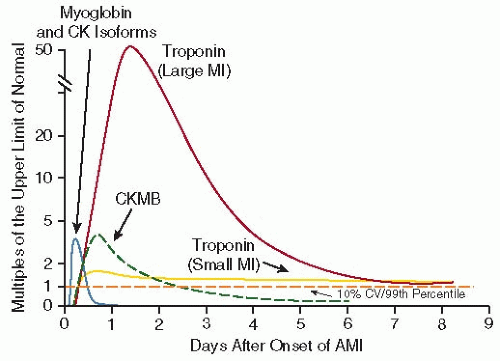
Table 90.4). The most widely used cardiac biomarkers currently are CK-MB and the cardiac troponins I and T (TnI and TnT). Myoglobin is rarely used clinically today. These proteins are found in high concentrations within the cardiac myocytes, making them specific for myocardial cell damage, and circulate at low levels within the peripheral circulation in the absence of cardiac myocyte necrosis (
FIGURE 90.2). The cardiac troponins are highly sensitive and specific for MI and may be detectable in the blood as soon as 2 hours after the onset of symptoms and may stay elevated for as long as 14 days after an infarct.
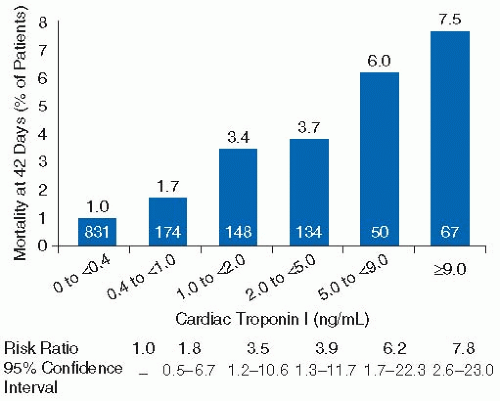
The elevation of cardiac biomarkers has important and well-validated prognostic significance beyond that obtainable from the ECG and the patient’s clinical parameters. An elevated troponin level is independently associated with an increased risk of death, and there is a direct and proportional relationship between the degree of elevation and this risk of death
45,46,47 (
FIGURE 90.3).
Risk Stratification in NSTEACS Using Risk Scores
Once other causes of the patient’s symptoms have been excluded and ACS is felt to be the diagnosis, early risk stratification is important to determining appropriate management. Doing this can aid in selecting the appropriate level of care needed for the patient, guiding the choices of the most appropriate medical therapies, and assisting in deciding between an early invasive strategy in the catheterization laboratory and a more conservative strategy initially with medical therapy.
Early risk stratification can help differentiate patients with NSTEMI and UA who often present with very heterogeneous symptoms. Multiple factors determine the likelihood of obstructive CAD and prognosis. Several risk scores have been developed to help clinicians identify high-risk patients rapidly. These include the Thrombolysis in Myocardial Infarction (TIMI) risk score and the Global Registry of Acute Coronary Events (GRACE) risk score (
FIGURE 90.4 and
Table 90.5).
48,49The TIMI risk score was developed as a simple tool comprising seven questions rating risk on presentation designed to assess patients with suspected UA or NSTEMI. For details on the score, see legend to
Table 90.5. The TIMI risk score provides an estimate of death, MI, or recurrent ischemia requiring urgent revascularization within 14 days from presentation. It has been validated in several studies and is a quick and relatively easy bedside tool to help clinicians stratify patients based on risk.
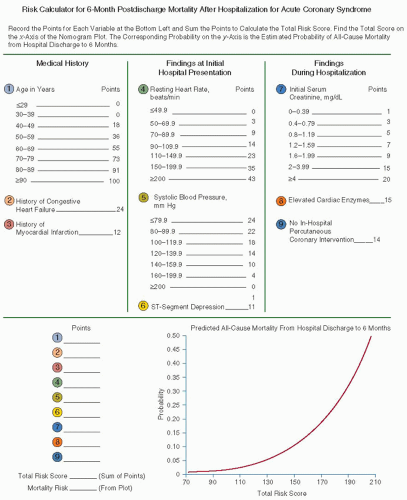
The GRACE prediction score is a model that can be used to predict in-hospital mortality and mortality from discharge to 6 months in patients presenting with ACS.
49,50 The GRACE model for predicting 6-month postdischarge mortality uses factors such as age, personal history of heart failure and MI, initial blood pressure and pulse, ECG, and laboratory findings (
FIGURE 90.4). It is a useful clinical tool for patients with NSTEMI, STEMI, and UA to predict 6-month mortality.
Initial Invasive Versus Initial Conservative Therapy
For patients presenting with NSTEMI or UA, one of two treatment pathways is usually decided upon based on patient risk and clinical stability. One pathway is labeled the “early invasive” strategy, while the other pathway for treatment is called the “initial conservative” or “selective invasive” strategy.
3 This terminology refers to the chosen angiographic strategy for the patient and the intended timing of diagnostic angiography with planned PCI. In both pathways, patients receive the usual
medical therapy for NSTEMI and UA that includes antianginal, antiplatelet, and anticoagulant therapy (
FIGURES 90.5 and
90.6).
In the early invasive strategy, patients usually undergo coronary angiography within 24 to 48 hours after admission. In the initial conservative strategy, patients do not undergo coronary angiography initially unless they have failed medical therapy or have documented ischemia, such as that demonstrated by a stress test, despite optimal medical therapy.
Deciding between an early invasive and conservative strategy is based largely on patient characteristics. High-risk features favor an early invasive strategy. These may include refractory ischemia, elevated cardiac biomarkers, dynamic ECG changes, hemodynamic or arrhythmic instability, prior revascularization, an elevated risk score (TIMI or GRACE), or left ventricular dysfunction (
Table 90.6). In addition, patients treated with an early invasive strategy include those without ST-segment elevation who undergo urgent cardiac catheterization due to clinical instability or refractory symptoms despite medical therapy. Patients at lower risk, or those with extensive comorbidities, where the risks of angiography may be considered to be too high, are typically treated with an initial conservative strategy.
For patients being treated with a conservative strategy, medical therapy is pursued with the intent of delaying the decision to proceed to coronary angiography until further clinical data are obtained. Generally in this group, the decision to go to diagnostic angiography is based upon results of a functional stress test showing objective evidence of ischemia, or upon persistent symptoms despite medical therapy.
The reasoning behind a conservative strategy is to avoid an invasive procedure and its inherent risk until the patient demonstrates objective evidence for ischemia or has not responded to medical therapy. The benefits to this strategy include the fact that many patients will stabilize on medical therapy and not require coronary angiography, thus reducing the use of an invasive and costly procedure. Patients treated in this pathway should have a stress test prior to discharge as well as an assessment for left ventricular function.
The early invasive strategy provides an angiographic method for risk stratification and a definitive evaluation for CAD. This strategy can differentiate those patients with no CAD from
those with severe CAD who may benefit from revascularization.
51,52 Furthermore, percutaneous revascularization of the culprit lesion may lead to a reduced risk for rehospitalization and reduced angina after discharge.
53Several trials have compared the early invasive strategy to the initial conservative approach. A meta-analysis of these trials concluded that a routine invasive approach is superior to a conservative strategy in reducing death or MI (14.4% vs. 12.2%;
OR = 0.82; 95% CI, 0.72 to 0.93).
54 This analysis showed an 18% relative reduction in death or MI, as well as a sustained reduction in mortality following discharge from the hospital with the invasive approach. In addition, the invasive strategy led to a reduction in angina, fewer hospitalizations, and improvements in quality of life.
A second meta-analysis of more contemporary randomized trials in NSTEMI also showed benefit with an early invasive approach.
55 The results showed a long-term improvement in mortality and morbidity with the invasive strategy, including a reduction in MI and rehospitalizations (
FIGURES 90.7 and
90.8). These improvements seem to be sustained for years after the event. An analysis from FRISC-II (FRagmin and fast Revascularization during InStability in Coronary artery disease-II) showed that the reduction in death or nonfatal MI is sustained for up to 5 years, and data from RITA-3 (Third Randomized Intervention Treatment of Angina) also demonstrated a reduction in death and MI at 5 years.
56,57Overall, the objective is to identify those patients who are most likely to benefit from an aggressive early invasive approach and minimize the exposure to potential harm in those less likely to benefit. The decision to proceed to early coronary angiography, versus a delayed and more conservative approach, is an individual one that should consider the wishes of the patient and family as well as the patient’s comorbidities. It must also be emphasized that ACS is a dynamic process where the clinical stability of a patient and the perceived benefit of an aggressive approach may change rapidly.