Michael Phillips
Acinetobacter Species
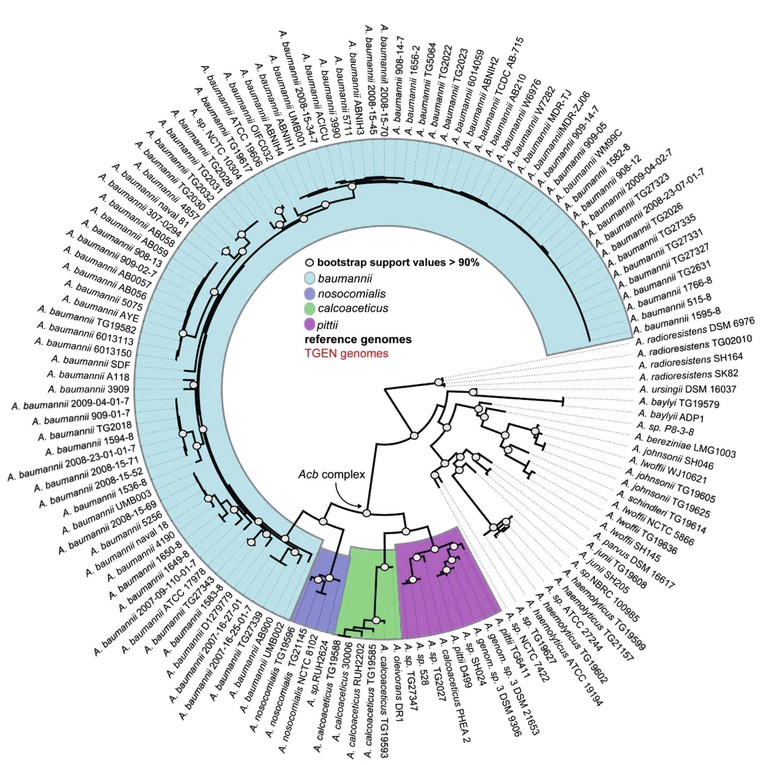
Acinetobacter, an aerobic, catalase-positive, oxidase-negative, gram-negative coccobacillus, was first described in 1911.1 Although ubiquitous in nature, the 23 named and more than 11 unnamed species of the genus Acinetobacter are associated with a specific ecologic niche that shapes their genomic contents.2,3 A. baumannii and the closely related and phenotypically indistinguishable A. pittii and A. nosocomialis are associated with health care infections, A. lwoffi and A. radioresistans colonize human skin and cause infection in immunocompromised hosts, A. calcoaceticus and A. johnsonii are prevalent in water and soil, and A. baylyi is frequently isolated from sewerage (Table 224-1).1,4–7 In the late 1980s, A. baumannii emerged as an important pathogen exhibiting increased antimicrobial resistance.8–10 Whole-genome sequencing analysis has suggested the rapid spread of multidrug-resistant A. baumannii was associated with a recently acquired ability to incorporate resistance determinants (Fig. 224-1).11–13 Multidrug-resistant A. baumannii was identified as an important pathogen after traumatic injuries sustained in soldiers during recent Middle Eastern conflicts; both environmental contamination during the injury and acquisition during medical care have been proposed as potential sources.14–16 This worldwide establishment of multidrug-resistant A. baumannii as a significant cause of health care–associated infections, coupled with a lack of potent antimicrobial agents in phase 2 or 3 of development, constitutes an important public health emergency.17
TABLE 224-1
Named Acinetobacter Species
| SPECIES NAME | TYPICAL HABITAT OR SOURCE |
| A. baumannii | Humans |
| A. baylyi | Sludge, soil |
| A. beijerinckii | Soil, water |
| A. bereziniae | Humans, soil |
| A. bouvetii | Sludge |
| A. calcoaceticus | Soil, water |
| A. gerneri | Sludge |
| A. grimontii | Sludge |
| A. guillouiae | Humans, water, soil |
| A. gyllenbergii | Humans |
| A. haemolyticus | Humans |
| A. johnsonii | Humans, water, soil |
| A. junii | Humans |
| A. lwoffii | Humans |
| A. nosocomialis | Humans |
| A. parvus | Humans, animals |
| A. pittii | Humans |
| A. radioresistens | Humans, soil |
| A. schindleri | Humans |
| A. soli | Soil |
| A. tandoii | Sludge, soil |
| A. tjernbergiae | Sludge |
| A. towneri | Sludge |
| A. ursingii | Humans |
| A. venetianus | Water |
Modified from Visca P, Seifert H, Towner KJ. Acinetobacter infection—an emerging threat to human health. IUBMB Life. 2011;63:1048-1054.
Epidemiology
Health Care–Associated Infections
Health care–associated infections represent the greatest public health impact of Acinetobacter, given the rapid spread of antibiotic-resistant strains and their continuing acquisition of additional resistance mechanisms. The application of molecular typing methods has revealed that a limited number of widespread clonal lineages of A. baumannii are responsible for hospital outbreaks worldwide.3 A. baumannii and the phenotypically indistinguishable A. nosocomialis and A. pittii cause the vast majority of health care–associated infections, but a review of clinical Acinetobacter isolates suggest A. lwoffii and A. ursingii may be emerging as possible pathogens.18 A. baumannii has increased in frequency as the cause of health care–associated pneumonia over the past 2 decades, to cause between 3% to 7% of cases.19,20 Among patients requiring mechanical ventilation for more than 5 days, Acinetobacter was the most frequent pathogen in one series, accounting for 26% of pneumonia cases.21 Acinetobacter also causes 1% to 2% of bloodstream infections associated with intravascular catheters, surgical site infections, and urinary tract infections.19 Less frequent health care–associated infections include meningitis after neurosurgery and wound infections in burn patients.22,23,24
Risk factors for A. baumannii colonization in the health care setting include residence in a nursing home, prolonged admission to an intensive care unit, and exposure to third-generation cephalosporins, fluoroquinolones, or carbapenems.25–30 The mortality associated with A. baumannii depends on infection type and underlying immunocompromise but is especially high in solid-organ transplant patients.31–34
The ability of Acinetobacter species to survive for weeks on surfaces within the hospital environment leads to prolonged outbreaks, and patient and staff movement between health care facilities results in regional spread.26,27,35–39 Essentially any moist or dry surface within a patient care area may become contaminated and serve as a reservoir for ongoing transmission, including sinks, faucets, humidifiers, hydrotherapy pools, curtains, pillows, bedrails, and equipment such as supply carts, infusion pumps, and equipment control touch pads.24,40–46 Patients with both recent and remote history of infection are able to contaminate their surrounding environment.46
Transmission of Acinetobacter within the health care setting occurs after lapses in proper hand hygiene and failure to disinfect mobile medical equipment and surfaces within patient care areas.47–49 Inadequate disinfection routines result in higher levels of environmental contamination and have been directly associated with patient colonization and subsequent Acinetobacter infection.50 Procedures that result in a spray of contaminated fluids, such as pulsatile lavage of wounds, may also lead to heavy environmental contamination.51 In addition to contaminated surfaces, airborne particles are believed to play a role in transmission of Acinetobacter, either by spread through open units with multiple beds or contamination of internal air filters of medical equipment.52–55 Air ionization has been proposed as a control method, which may either directly affect the bacteria or repel the dust containing Acinetobacter by changing the electrostatic characteristics of plastic items of medical equipment.56,57 An increase in health care–associated Acinetobacter infections during the warmer, more humid months has been reported, potentially owing to contamination of air handling systems.58,59
Timely investigation of outbreaks requires the combination of traditional epidemiologic surveillance with molecular techniques to help identify potential routes of transmission and implement control measures.60–62 Molecular genotyping may also reveal changes in the predominant strain of multidrug-resistant A. baumannii causing health care–associated outbreaks over time.63,64 Outbreak strains are typically more resistant to antibiotics and may be associated with specific clinical syndromes, highlighting the importance of molecular epidemiology.65–67
Community-Associated Infections
Several case series describe a syndrome of severe community-acquired A. baumannii pneumonia, which typically presents during the warm, humid months in tropical regions.68–70 The case-fatality rate is higher than for hospital-acquired infections, with most patients presenting in respiratory failure and shock.69,70 Community-acquired Acinetobacter pneumonia in foundry workers has also been reported; air sampling in the workplace isolated the same strain. The presence of chronic inflammation from inhaled iron particles or increased Acinetobacter virulence in the presence of iron are potential reasons for the increased susceptibility to infection in these individuals.71–73 Finally, community-acquired Acinetobacter meningitis in patients without underlying medical compromise has been rarely reported; outcomes in those who received prompt therapy were favorable.74
Trauma
A. baumannii skin and soft tissue infections have been associated with traumatic injuries suffered during warfare and natural disasters.75,76,77 The source of A. baumannii in these infections is unclear; inoculation at the time of trauma and health care exposure during medical treatment in mobile or temporary medical treatment facilities are both potential mechanisms.78–80 The subsequent transfer of patients infected or colonized with multidrug-resistant Acinetobacter may serve as the source for outbreaks at receiving health care facilities.81,82
Diagnosis
Acinetobacter is readily isolated on standard culture media, but differentiation of species based on phenotype alone is difficult, leading to the term A. calcoaceticus–A. baumannii complex.83 Newer methodologies incorporating matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry rapidly identify both antimicrobial resistance mechanisms and specific clones of Acinetobacter species and may allow timely selection of appropriate therapy and implementation of targeted infection control efforts.84–86 Quantitative real-time polymerase chain reaction assay of the DNA encoding the intrinsic β-lactamase OXA 51 may also prove useful as a rapid diagnostic test of A. baumannii bacteremia, but its ability to measure treatment efficacy requires further study.87,88
Clinical Manifestations
Pneumonia associated with hospitalization or mechanical ventilation is a frequent and important clinical manifestation of Acinetobacter infection, resulting in greater mortality and prolonged length of hospital stay.89 When Acinetobacter is isolated from a pulmonary specimen, differentiation between colonization and pneumonia is critically important and may be difficult, requiring careful clinical correlation. Mortality is increased in those with pneumonia and underlying comorbidities or associated bacteremia.90 A. baumannii was independently associated with increased mortality when compared with A. nosocomialis health care–associated pneumonia complicated by bacteremia in one series, in spite of their close genetic relatedness.91 As discussed earlier, severe community-associated A. baumannii pneumonia with a high fatality rate is reported during warm, humid months in tropical regions.69,70
Acinetobacter species account for 1% to 2% of all bloodstream infections and are typically associated with intravascular devices, with 63% of those infections caused by A. baumannii, followed by A. nosocomialis (20%) and A. pittii (8%) in one series of 295 bloodstream isolates.92 The mortality of those with A. baumannii bacteremia was significantly higher than those with A. nosocomialis and A. pittii bacteremia (37% vs. 16% and 14%, P < .001); other species such as A. lwoffii and A. junii have low bacteremia-associated mortality.92,93,94 A. lwoffii bacteremia typically occurs in patients with underlying malignancy.94
Acinetobacter causes about 1% of urinary tract infections, most of which involve strains with the ability to form biofilms on urinary catheters.19,95 Outbreaks of A. baumannii meningitis in postsurgical neurosurgery patients have been reported; the incidence decreased with antibiotic restriction and implementation of infection control measures.22,23 Community-associated Acinetobacter meningitis in patients without surgical procedures has rarely been reported.74 A. baumannii skin and soft tissue infections after traumatic injuries and burns are associated with exposures within the health care setting.24,77
Pathogenesis and Antimicrobial Resistance
The pathogenicity of A. baumannii relates to its ability to adhere to surfaces utilizing pili, to create biofilm on surfaces and human cells, to survive in iron-limited environments within the host, and to acquire foreign genetic material to enhance survival and develop large repertoires of antibiotic resistance mechanisms.45,72,96–99,100 The production of Acinetobacter biofilm-associated protein and the presence of a major facilitator superfamily transporter within the bacterial cell wall are associated with biofilm formation and adherence to host cells.2,101,102 Neutrophils, recruited by natural killer cells, play the predominant role in host immune response to Acinetobacter infection.103–105 Different A. baumannii strains elicit a range of innate immune responses and affect their ability to survive within the host.106,107
Acinetobacter is well known for its multitude of antimicrobial resistance mechanisms, which are associated with an increase in genome size (Fig. 224-2).12,108 Genomic analysis reveals pathogenic strains of A. baumannii that contain genes clustered on resistance islands, whose structure may facilitate the acquisition of resistance mechanisms from other species of bacteria.109 Insertion sequences such as ASAba1 within the Acinetobacter genome promote the expression of neighboring genes and result in the overexpression of several key resistance mechanisms. Finally, it is postulated that the ability of Acinetobacter to acquire resistance determinants more effectively than other bacteria is due to the close association of several Acinetobacter species to the soil and water environment that contain a large reservoir of resistance genes.110
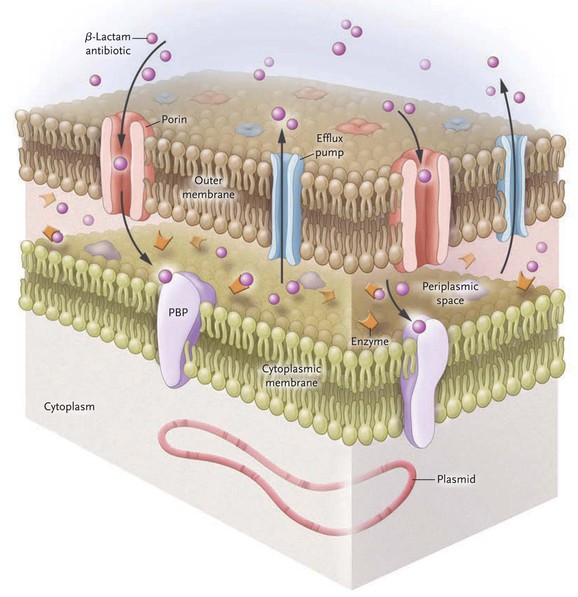
Acinetobacter species exert much of their antibiotic resistance through the expression of β-lactamases. Group 1 Ambler class C β-lactamases are chromosomally encoded cephalosporinases that hydrolyze penicillins and first-, second-, and third-generation cephalosporins, including ceftazidime, cefotaxime, and ceftriaxone. Rates of hydrolysis of fourth-generation cephalosporins, such as cefepime, and carbapenems, by class C enzymes are low.111 The class C β-lactamases are not inducible in A. baumannii, but insertion of promoter sequence ASAba1 increases expression.112 Groups 2b and 2c Ambler class A β-lactamases are encoded on genes carried by large plasmids and confer resistance to penicillins and narrow-spectrum cephalosporins.113 The emergence and rapid worldwide spread of the group 2be Ambler class A extended-spectrum β-lactamases (ESBLs) in the 1980s illustrated how drug-resistant strains of A. baumannii spread rapidly. These ESBLs exhibit hydrolytic activity over penicillins and all cephalosporins, thereby promoting the use of carbapenems to treat for significant Acinetobacter infection. The subsequent emergence of the Ambler class D oxacillinases group 2d has largely been responsible for the increase in carbapenem resistance seen since 2000.114,115 The insertion sequence ISAba1 is a promoter for genes encoding oxacillinases in Acinetobacter.116,117 The oxacillinase OXA-51-like is intrinsic and chromosomal, whereas the acquired OXA subclasses, called carbapenem-hydrolyzing class D β-lactamases, are found both on chromosomes and on plasmids. Examples of the acquired OXA enzymes include 23-like, 24 (33-like, 40-like), 58-like, 143-like, and 235-like, which may be derived from other Acinetobacter species.114,118,119 The group 3 Ambler class B metallo-β-lactamases are a less-frequent but emerging cause of carbapenem resistance. The metallo-β-lactamases IMP and VIM are more common and widespread, but NDM-1 and NDM-2 have been identified in multiple countries and will likely disseminate.120–125,126
The second most important determinant of drug resistance in Acinetobacter is the presence of efflux pumps, which confer resistance to β-lactam antibiotics, chloramphenicol, macrolides, tetracyclines, tigecycline, aminoglycosides, and certain antiseptics. Efflux pumps in the resistance-nodulation division (RND) family are located on chromosomes and are overexpressed after genetic mutation. The predominant pump expressed in this family is AdeABC, although others have been described.127,128 Non-RND efflux systems seen in Acinetobacter are encoded by mobile genetic elements; eight efflux genes are clustered on the resistance island AbaR1 in the AYE strain of A. baumannii implicated in multiple health care–associated outbreaks.109 Antiseptic resistance is also encoded by efflux pumps; a small drug resistance (SMR) efflux pump may confer quaternary ammonium resistance, and RND efflux pumps result in biocide resistance.129,130
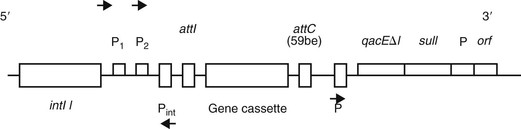
Aminoglycoside resistance in Acinetobacter species is determined by the presence of aminoglycoside-modifying enzymes (AMEs). These enzymes phosphorylate, acetylate, or adenylate the aminoglycoside molecule and decrease their binding affinity to the ribosomal subunit. AMEs are encoded on genetically mobile elements, especially class 1 integrons, which frequently also contain genetic elements for ESBLs and metallo-β-lactamases (Fig. 224-3).113,131
The expression of porins modifies the ability of antimicrobial agents to permeate the outer membrane of the bacterial cell wall; OmpAab is the principal outer membrane protein (Omp) in Acinetobacter and confers resistance to β-lactams and carbapenems. OmpAab has been characterized in A. radioresistens, A. junii, and A. baumannii.132,133 The presence of porins has been postulated as the cause of carbapenem heteroresistance detected by Etest in a small number of A. baumannii isolates; these heteroresistant strains were identical to their original carbapenem-susceptible isolates when analyzed by pulsed-field gel electrophoresis but differed phenotypically by exhibiting high-level carbapenem resistance.134
Acinetobacter resistance to fluoroquinolones is multifactorial. Mutations in the quinolone resistance determining region lowers fluoroquinolone binding to bacterial DNA gyrase and topoisomerase IV; when combined with upregulation of the AdeABC efflux pump, quinolone resistance results.135–137
Resistance to the polymyxins, which include colistin (colistimethate sodium) and polymyxin B, is associated with both mutations in the genes encoding the two-component regulatory system PmrA and PmrB and a reduction in lipopolysaccharides in the Acinetobacter cell wall, resulting in less negative charge and loss of antimicrobial affinity.138,139 As with carbapenems, colistin heteroresistance has been identified in Acinetobacter and presents a concern for the emergence of resistance during treatment.138 Current evidence, however, suggests that colistin-resistant strains of A. baumannii are less fit, which may potentially slow the spread of this pan-resistant strain.140,141
Therapy
The wide range of acquired resistance mechanisms in Acinetobacter dictates that the choice of empirical therapy must be based on individual patient risk for acquisition of resistant strains and on the local epidemiology. Before initiation of therapy, determination of whether the Acinetobacter strain isolated from culture represents invasive infection or merely colonization is needed to avoid antibiotic overutilization, given the bacteria’s ability to form biofilms and to colonize surfaces.
β-Lactam Antibiotics
When the organisms are susceptible, β-lactam antibiotics are the drugs of choice for Acinetobacter infections because they are rapidly bactericidal and distribute widely throughout the body. Intrinsic Acinetobacter β-lactamases, however, inactivate first- and second-generation cephalosporins and penicillins, and the widespread distribution of acquired β-lactamases renders third- and fourth-generation cephalosporins, such as cefepime, ceftriaxone, and cefotaxime, viable options only if susceptible, based on in vitro testing.1,142,143 These resistance enzymes resulted in the carbapenems emerging as the drugs of choice for hospital-acquired Acinetobacter infections.144–146 When interpreting resistance testing, manual testing may be more accurate than automated systems and each carbapenem must be considered separately given clinically relevant discordance between agents in this class.147,148 Unfortunately, the worldwide spread of virulent carbapenem-resistant strains has threatened the effectiveness of all β-lactams for treatment of Acinetobacter infections.126,149
β-Lactamase Inhibitors
With the emergence of carbapenem-resistant Acinetobacter strains, the use of β-lactamase inhibitors has emerged as a therapeutic option. Sulbactam has the highest activity of β-lactamase inhibitors.150 Sulbactam alone or combined with ampicillin is effective for invasive infections, including pneumonia, bloodstream infections, and meningitis.151–153 Sulbactam is equally effective in treating pneumonia and bacteremia compared with carbapenem and polymyxin B.154–157 A dose of at least 6 g/day of sulbactam in divided doses is recommended for most infections in adults, although doses as high as 9 g/day in divided doses have been utilized.146,155 New β-lactamase inhibitors are being developed.
Aminoglycosides
Although susceptibility testing may show isolates of Acinetobacter are susceptible to aminoglycosides, the use of these agents is limited owing to low penetration into the lungs and central nervous system, concern that automated tests for Acinetobacter susceptibility to aminoglycosides may be inaccurate, and inferiority of aminoglycoside monotherapy against gram-negative bacterial infections outside the urinary tract.158–162 Acinetobacter susceptibility to aminoglycosides varies by drug type, with tobramycin being the most active.158,163
Tigecycline and Minocycline
Tigecycline, a member of the glycylcycline class of antibiotics, has been successfully used to treat carbapenem-resistant Acinetobacter infections, but these results are based on observational studies and the drug is frequently given in combination with other antibiotics.164,165–167 Additional concerns include the large volume of distribution and low serum concentration of tigecycline, which precludes its use for bloodstream infections, and reports of the development of resistance while on therapy.166,168,169,170 Minocycline, a tetracycline derivative, has in vitro activity against carbapenem-resistant Acinetobacter isolates, and combination with colistin has shown bactericidal activity.81,171,172
Polymyxins
Colistin and polymyxin B are frequently included in the treatment of carbapenem-resistant Acinetobacter infections. Although the optimal dosing of these antibiotics to treat Acinetobacter infection has not been determined by randomized trials, recent evidence suggests that a loading dose followed by high doses with longer dosing intervals may improve outcomes.173–176 Reserving use of these agents for β-lactam–resistant infections is recommended, given the inferior outcomes when colistin is compared with β-lactam antibiotics.177 Although colistin resistance in A. baumannii may occur sporadically, most reports identify an association with prior colistin use, raising concerns of the emergence of resistance on therapy.178 Innate colistin resistance is common in certain Acinetobacter species such as A. junii.179 In areas with a high prevalence of carbapenem- and sulbactam-resistant Acinetobacter, polymyxins should be considered as a component of empirical therapy for severe infection.180–182
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree