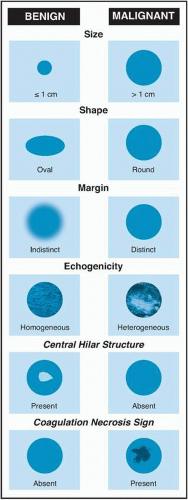
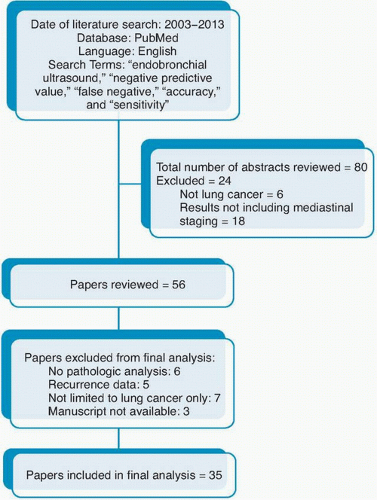
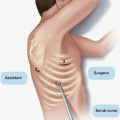
Author, Year |
Study Design |
No. of Patients |
Prevalence of N2 Disease (%) |
NPV |
Sensitivity |
Accuracy |
Key Findings |
Potential for Significant Bias |
Cornwell et al, 20133 |
Retrospective |
62 |
5 |
93 |
67 |
94 |
In patients with clinical stage I NSCLC, EBUS results in a lower incidence of nontherapeutic thoracotomy than noninvasive staging does, but this difference is not significant. |
Yes |
Herth et al, 20084 |
Prospective observational |
97 |
10 |
98.9 |
89 |
NA |
EBUS is accurate in NSCLC staging for patients with clinical stage I NSCLC determined by CT and PET findings. |
Yes |
Herth et al, 20065 |
Prospective observational |
100 |
121 |
96.3 |
92.3 |
NA |
EBUS is beneficial in patients with clinical stage I NSCLC. It prevents nontherapeutic thoracotomy in 1 of 6 patients. PET was not used routinely. |
Yes |
Hwangbo et al, 20096 |
Prospective observational |
126 |
26 |
96.7 |
90 |
97.4 |
EBUS is useful for confirming N2 disease detected by PET. It is also useful for detecting N2 disease in patients with radiographic N0 disease. |
Yes |
Lee et al, 20087 |
Retrospective |
102 |
30 |
96.9 |
93.8 |
97.9 |
Optimal results with EBUS are obtained when at least 3 aspirations of each lymph node are performed. |
Yes |
Yasufuku et al, 20118* |
Prospective controlled trial |
153 |
35 |
91 |
81 |
93 |
EBUS is equivalent to mediastinoscopy in the mediastinal staging of NSCLC. |
No |
Feller-Kopman et al, 20099* |
Retrospective |
131 |
35 |
89.7 |
85 |
NA |
EBUS is an accurate and sensitive method for diagnosing and staging NSCLC. |
Yes |
Petersen et al, 200910 |
Retrospective |
157 |
43 |
90 |
85 |
NA |
EBUS is accurate in staging the mediastinum in NSCLC patients. The routine confirmation of negative EBUS findings with mediastinoscopy has a minor role in NSCLC staging. |
Yes |
Sanz-Santos et al, 201211 |
Retrospective |
296 |
51 |
93.6 |
NA |
NA |
EBUS can be used to sample lymph node regions 4R, 4L, and 7 in more than 80% of patients. In such patients, EBUS has an NPV of >90% for mediastinal malignancy. |
Yes |
Nakajima et al, 201312* |
Retrospective |
438 |
52 |
90 |
97 |
98 |
ROSE during EBUS results in a low incidence of nondiagnostic samples. |
Yes |
Jhun et al, 201213 |
Retrospective |
151 |
55 |
84.3 |
91.6 |
93.8 |
The diagnostic yield of EBUS is lower for left paratracheal lymph nodes. The diagnostic yield is not related to lymph node size. |
Yes |
Szlubowski et al, 200914 |
Retrospective |
226 |
57 |
89 |
83.5 |
92.9 |
EBUS is an effective and safe technique for mediastinal staging in NSCLC patients. In patients with negative EBUS results, surgical exploration of the mediastinum should be performed. |
Yes |
Bauwens et al, 200815 |
Retrospective |
106 |
58 |
91 |
95 |
97 |
EBUS is a reasonable first step in the confirmation of N2 disease in NSCLC patients. Surgical mediastinal staging should be used to confirm negative EBUS findings. |
Yes |
Joesph et al, 201316* |
Retrospective |
131 |
58 |
90 |
92 |
NA |
ROSE does not affect clinical decisions made during staging EBUS. |
Yes |
Lee et al, 201217 |
Retrospective |
73 |
62 |
94 |
95 |
97 |
EBUS can be used to accurately assess the mediastinum in patients with NSCLC and radiographic N2 disease. |
Yes |
Cerfolio et al, 201018 |
Retrospective |
72 |
63 |
79 |
57 |
83 |
EBUS and EUS have high false negative rates, and negative results should be confirmed prior to thoracotomy. |
Yes |
Navani et al, 201219 |
Retrospective |
774 |
65 |
88 |
72 |
NA |
EBUS samples are suitable for use in NSCLC subtyping and EGFR mutation analysis. |
Yes |
Kuo et al, 201120 |
Retrospective |
43 |
65 |
85.7 |
80.6 |
91 |
The diagnostic accuracy of EBUS is higher than that of PET in a tuberculosis-endemic population. |
Yes |
Hu et al, 201321 |
Retrospective |
231 |
67‡ |
92 |
88 |
87 |
Proficiency using EBUS requires 22 cases. Lymph node size is a predictor of success. |
Yes |
Yasufuku et al, 200522* |
Prospective observational |
105 |
67 |
89.5 |
94.6 |
96.3 |
EBUS is an accurate staging procedure in patients with NSCLC. |
Yes |
Rintoul et al, 200923 |
Retrospective |
109 |
71 |
60 |
91 |
92 |
EBUS can be used to accurately evaluate PET-positive hilar and mediastinal lymph nodes. Negative findings should be confirmed by surgical means. |
Yes |
Cetinkaya et al, 201124 |
Retrospective |
52 |
80 |
83 |
95 |
96 |
EBUS is safe and accurate in NSCLC staging. |
Yes |
Ernst et al, 200825 |
Prospective cross-over |
60 |
89 |
78 |
87 |
NA |
The difference between EBUS and mediastinoscopy in determining the N status of patients with NSCLC is not statistically significant. |
Yes |
Gu et al, 200926 |
Meta-analysis |
1,299 |
NA |
93 |
NA |
NA |
EBUS has a high NPV and is costeffective in the mediastinal staging of NSCLC. |
|
Adams et al, 200927 |
Meta-analysis |
782 |
NA |
NA |
88 |
NA |
EBUS has high sensitivity in the mediastinal staging of NSCLC. |
|
Abu-Hijleh et al, 201328* |
Retrospective |
200 |
NA |
75 |
87 |
91 |
EBUS is similar to surgical staging in patients with NSCLC. The NPV of EBUS is highest after the initial 25-50 cases. The accuracy of EBUS is independent of lymph node size or location and number of passes. |
|
Dong et al, 201329* |
Meta-analysis |
1,066 |
NA |
93 |
90 |
96 |
EBUS is accurate and safe in staging NSCLC. |
|
Whitson et al, 201330* |
Retrospective |
120 |
NA |
66†
85 |
83†
93 |
87†
95 |
The inclusion of nondiagnostic results yields a lower NPV, sensitivity, and accuracy. |
|
* ROSE was used.
† For the Whitson study, the first set of numbers includes nondiagnostic specimens. The values for when nondiagnostic studies are included are shown below in the same field.
‡ Incidence of N1 and N2 disease. |
CT, computed tomography; EBUS, endobronchial ultrasonography; EGFR, epidermal growth factor receptor; NA, not available; NPV, negative predictive value; NSCLC, non-small cell lung cancer; PET, positron emission tomography; ROSE, rapid on-site evaluation. |