In postnatal life, the bone marrow and thymus are the primary lymphoid organs in which lymphocytes develop (Fig. 9.2). The secondary lymphoid organs in which specific immune responses are generated are the lymph nodes, spleen and lymphoid tissues of the alimentary and respiratory tracts.
Figure 9.2 Primary and secondary lymphoid organs and blood. TdT, terminal deoxynucleotidyl.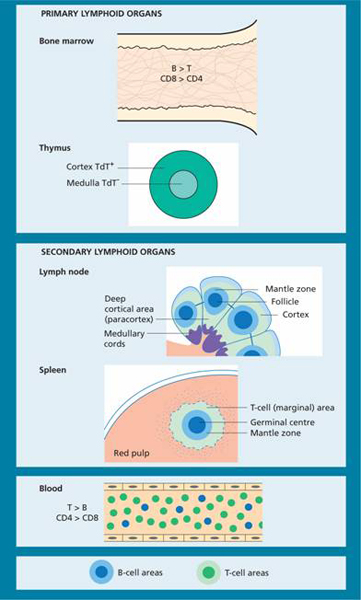
B and T lymphocytes
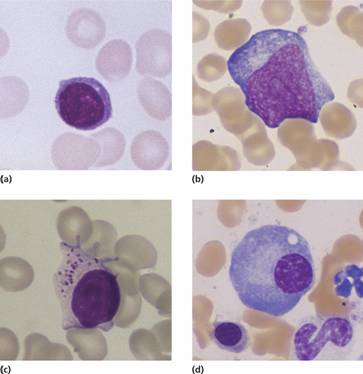
The immune response depends upon two types of lymphocytes, B and T cells (Table 9.1), which derive from the haemopoietic stem cell. B cells mature in the bone marrow and circulate in the peripheral blood until they undergo recognition of antigen. The B-cell receptor is membrane-bound immunoglobulin and after activation this is secreted as free soluble immunoglobulin. At this point they mature into memory B cells or plasma cells. The latter home to the bone marrow and have a characteristic morphology with an eccentric round nucleus with a ‘clock-face’ chromatin pattern and strongly basophilic cytoplasm (Fig. 9.1d).
Table 9.1 Functional aspects of T and B cells.
| T cells | B cells | |
| Origin | Thymus | Bone marrow |
| Tissue distribution | Parafollicular areas of cortex in nodes, periarteriolar in spleen | Germinal centres of lymph nodes, spleen, gut, respiratory tract; also subcapsular and medullary cords of lymph nodes |
| Blood | 80% of lymphocytes; CD4 > CD8 | 20% of lymphocytes |
| Membrane receptor | TCR for antigen | BCR (= immunoglobulin) for antigen |
| Function | CD8+: CMI against intracellular organisms | Humoral immunity by generation of antibodies |
| CD4+: T-cell help for antibody production and generation of CMI | ||
| Characteristic surface markers | CD1 | CD19 |
| CD2 | CD20 | |
| CD3 | CD22 | |
| CD4 or 8 | CD9 (pre B cells) | |
| CD5 | CD10 (precursor B cells) | |
| CD6 | CD79 a and b | |
| CD7 | HLA class I and II | |
| HLA class I | ||
| HLA class II when activated | ||
| Genes rearranged | TCR α, β, γ, δ | IgH, Igκ, Igλ |
BCR, B-cell receptor; C, complement; CMI, cell-mediated immunity; HLA, human leucocyte antigen; Ig, immunoglobulin; TCR, T-cell receptor.
T cells develop from cells that have migrated to the thymus where they differentiate into mature T cells during passage from the cortex to the medulla. During this process, self-reactive T cells are deleted (negative selection) whereas T cells with some specificity for host human leucocyte antigen (HLA) molecules are selected (positive selection). The mature helper cells express CD4 and cytotoxic cells express CD8 (Table 9.1). The cells also express one of two T-cell antigen receptor heterodimers, αβ (> 90%) or γδ (<10%), and recognize antigen only when it is presented at a cell surface (see below).
Natural killer cells
Natural killer (NK) cells are cytotoxic CD8+ cells that lack the T-cell receptor (TCR). They are large cells with cytoplasmic granules and typically express surface molecules CD16 (Fc receptor), CD56 and CD57. NK cells are designed to kill target cells that have a low level of expression of HLA class I molecules such as may occur during viral infection or on a malignant cell. NK cells do this by displaying a number of receptors for HLA molecules on their surface. When HLA is expressed on the target cell these deliver an inhibitory signal into the NK cell. When HLA molecules are absent on the target cell this inhibitory signal is lost and the NK cell can then kill its target. In addition, NK cells display antibody-dependent cell-mediated cytotoxicity (ADCC). In this, antibody binds to antigen on the surface of the target cell and then NK cells bind to the Fc portion of the bound antibody and kill the target cell.
Lymphocyte circulation
Lymphocytes in the peripheral blood migrate through post-capillary venules into the substance of the lymph nodes or into the spleen or bone marrow. T cells home to the perifollicular zones of the cortical areas of lymph nodes (paracortical areas) (Fig. 9.2) and to the periarteriolar sheaths surrounding the central arterioles of the spleen. B cells selectively accumulate in follicles of the lymph nodes and spleen. Lymphocytes return to the peripheral blood via the efferent lymphatic stream and the thoracic duct. CD4 helper cells predominate in normal peripheral blood and germinal centres, but in the marrow and gut the major T-cell subpopulation is CD8 positive.
These are a group of proteins produced by plasma cells and B lymphocytes that bind to antigen. They are divided into five subclasses or isotypes: immunoglobulin G (IgG), IgA, IgM, IgD and IgE. IgG, the most common, contributes approximately 80% of normal serum immunoglobulin and is further subdivided into four subclasses: IgG1, IgG2, IgG3 and IgG4. IgA is subdivided into two types.
IgM is usually produced first in response to antigen, IgG subsequently and for a more prolonged period. The same cell can switch from IgM to IgG, or to IgA or IgE synthesis. IgA is the main immunoglobulin in secretions, particularly of the gastrointestinal tract. IgD and IgE (involved in delayed hypersensitivity reactions) are minor fractions. Some important properties of the three main immunoglobulin subclasses are summarized in Table 9.2.
Table 9.2 Some properties of the three main classes of immunoglobulin (Ig).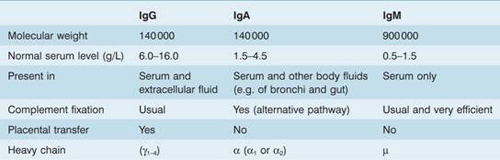
The immunoglobulins are all made up of the same basic structure (Fig. 9.3) consisting of two heavy chains which are called gamma (γ) in IgG, alpha (α) in IgA, mu (μ) in IgM, delta (δ) in IgD and epsilon (ε) in IgE, and two light chains–kappa (κ) or lambda (λ)–which are common to all five immunoglobulins. The heavy and light chains each have highly variable regions which give the immunoglobulin specificity, and constant regions in which there is virtual complete correspondence in amino acid sequence in all antibodies of a given isotype (e.g. IgA, IgG) or isotype subclass (e.g. IgG1, IgG2). IgG antibody can be broken into a constant Fc fragment and two highly variable Fab fragments. IgM molecules are much larger because they consist of five subunits.
Figure 9.3 Basic structure of an immunoglobulin molecule. Each molecule is made up of two light (κ or λ) (blue areas) and two heavy (purple) chains, and each chain is made up of variable (V) and constant (C) portions, the V portions including the antigen-binding site. The heavy chain (μ, δ, γ, ε or α) varies according to the immunoglobulin class. IgA molecules form dimers, while IgM forms a ring of five molecules. Papain cleaves the molecules into an Fc fragment and two Fab fragments.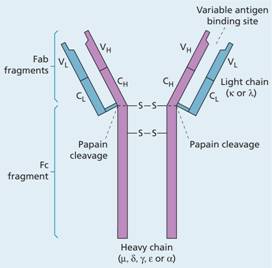
The main role of immunoglobulins is defence of the body against foreign organisms. However, they also have a vital role in the pathogenesis of a number of haematological disorders. Secretion of a specific immunoglobulin from a monoclonal population of lymphocytes or plasma cells causes paraproteinaemia (see p. 273). Bence-Jones protein found in the urine in some cases of myeloma consists of a monoclonal secretion of light chains or light-chain fragments (either κ or λ). Immunoglobulins may bind to blood cells in a variety of immune disorders and cause their agglutination (e.g. in cold agglutinin disease; see p. 84) or destruction following direct complement lysis or after elimination by the reticuloendothelial system.
Antigen–receptor gene arrangements
Immunoglobulin gene rearrangements
The immunoglobulin heavy-chain and κ and λ light-chain genes occur on chromosomes 14, 2 and 22, respectively. In the germline state, the heavy-chain gene consists of separate segments for variable (V), diversity (D), joining (J) and constant (C) regions. Each of the V, D and J regions contain a number (n) of different gene segments (Fig. 9.4). In cells not committed to immunoglobulin synthesis these gene segments remain in their separate germline state. During early differentiation of B cells there is rearrangement of heavy-chain genes so that one of the V heavy-chain segments combines with one of the D segments, which has itself already combined with one of the J segments. Thus, they form a transcriptionally active gene for the heavy chain. The protein coding segments of the C region mRNA are joined to the V region after splicing out intervening RNA. The class of immunoglobulin that is secreted depends on which of the nine (4γ, 2α, 1μ, 1δ and 1ε) constant regions is used.
Figure 9.4 Rearrangement of a heavy-chain immunoglobulin gene. One of the V segments is brought into contact with a D, a J and a C (in this case C μ) segment, forming an active transcriptional gene from which the corresponding mRNA is produced. The DJ rearrangement precedes VDJ joining. The class of immunoglobin depends on which of the nine constant regions (1μ, 1δ, 4γ, 2α, 1ε) is used.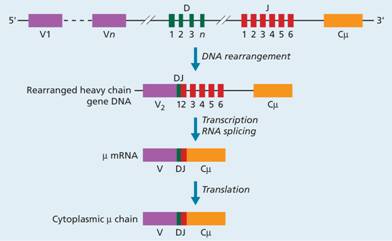
Diversity is introduced by the variability of which V segment joins with which D and with which J segment. In the arbitrary example shown in Fig. 9.4, V2 joins with D1 and J2. Additional diversity is generated by the enzyme terminal deoxynucleotidyl transferase (TdT), which inserts a variable number of new bases into the DNA of the D region at the time of gene rearrangement. Similar rearrangements occur during generation of the light-chain gene (Fig. 9.5). Enzymes called recombinases
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


