Introduction to macrocytic anaemia
In macrocytic anaemia the red cells are abnormally large (mean corpuscular volume, MCV > 98 fL). There are several causes (see Table 2.4) but they can be broadly subdivided into megaloblastic and non-megaloblastic (Table 5.10), based on the appearance of developing erythroblasts in the bone marrow.
This is a group of anaemias in which the erythroblasts in the bone marrow show a characteristic abnormality – maturation of the nucleus being delayed relative to that of the cytoplasm. The underlying defect accounting for the asynchronous maturation of the nucleus is defective DNA synthesis and this is usually caused by deficiency of vitamin B12 or folate. Less commonly, abnormalities of metabolism of these vitamins or other lesions in DNA synthesis may cause an identical haematological appearance (Table 5.1).
Table 5.1 Causes of megaloblastic anaemia.
| Vitamin B12 deficiency |
| Folate deficiency |
| Abnormalities of vitamin B12 or folate metabolism (e.g. transcobalamin deficiency, nitrous oxide, antifolate drugs) |
| Other defects of DNA synthesis |
| Congenital enzyme deficiencies (e.g. orotic aciduria) |
| Acquired enzyme deficiencies (e.g. alcohol, therapy with hydroxyurea, cytosine arabinoside) |
This vitamin is synthesized in nature by microorganisms; animals acquire it by eating other animal foods, by internal production from intestinal bacteria (not in humans) or by eating bacterially contaminated foods. The vitamin consists of a small group of compounds, the cobalamins, which have the same basic structure, with a cobalt atom at the centre of a corrin ring which is attached to a nucleotide portion (Fig. 5.1). The vitamin is found in foods of animal origin such as liver, meat, fish and dairy produce but does not occur in fruit, cereals or vegetables. Table 5.2 compares nutritional aspects of B12 and folate.
Figure 5.1 The structure of methylcobalamin (methyl B12), the main form of vitamin B12 in human plasma. Other forms include deoxyadenosylcobalamin (ado B12), the main form in human tissues; hydroxocobalamin (hydroxo B12), the main form in treatment.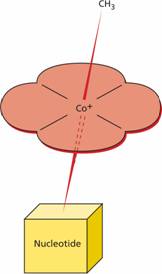
Absorption
A normal diet contains a large excess of B12 compared with daily needs (Table 5.2). B12 is released from protein-binding in food and is combined with the glycoprotein intrinsic factor (IF) (molecular weight 45 000) which is synthesized by the gastric parietal cells. The IF–B12 complex can then bind to a specific surface receptor for IF, cubilin, which then binds to a second protein, amnionless, which directs endocytosis of the cubilin IF–B12 complex in the distal ileum where B12 is absorbed and IF destroyed (Fig. 5.2).
Table 5.2 Vitamin B12 and folate: nutritional aspects.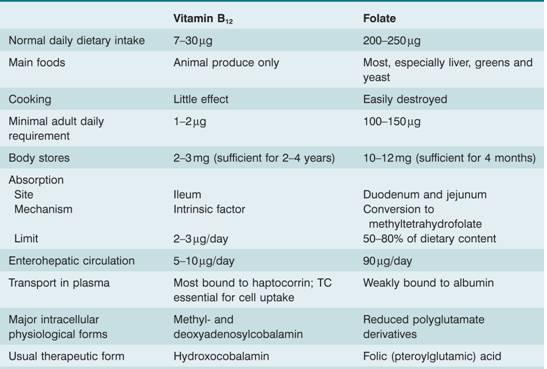
TC, transcobalamin; haptocorrin = transcobalamin 1.
Figure 5.2 The absorption of dietary vitamin B12 after combination with intrinsic factor (IF), through the ileum. Folate absorption occurs through the duodenum and jejunum after conversion of all dietary forms to methyl-tetrahydrofolate (methyl THF). TC, transcobalamin.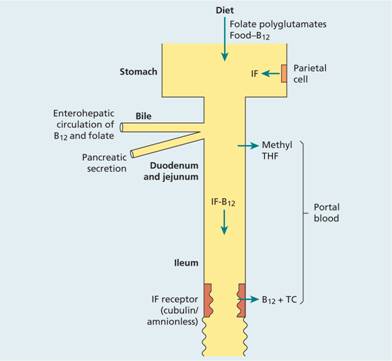
Transport: the transcobalamins
Vitamin B12 is absorbed into portal blood where it becomes attached to the plasma-binding protein transcobalamin (TC, previously called transcobalamin II) which delivers B12 to bone marrow and other tissues. Although TC is the essential plasma protein for transferring B12 into the cells of the body, the amount of B12 on TC is normally very low (< 50 ng/L). TC deficiency causes megaloblastic anaemia because of failure of B12 to enter marrow (and other cells) from plasma but the serum B12 level in TC deficiency is normal. This is because most B12 in plasma is bound to another transport protein, haptocorrin (previously called transcobalamin I). This is a glycoprotein largely synthesized by granulocytes and macrophages. In myeloproliferative diseases where granulocyte production is greatly increased, the haptocorrin and B12 levels in serum both rise considerably. B12 bound to haptocorrin does not transfer to marrow; it appears to be functionally ‘dead’. Closely related glycoproteins to plasma haptocorrin are present in gastric juice, milk and other body fluids.
Biochemical function
Vitamin B12 is a coenzyme for two biochemical reactions in the body: first, as methyl B12 it is a cofactor for methionine synthase, the enzyme responsible for methylation of homocysteine to methionine using methyl tetrahydrofolate (methyl THF) as methyl donor (Fig. 5.3a); and, secondly, as deoxyadenosyl B12 (ado B12) it assists in conversion of methylmalonyl coenzyme A (CoA) to succinyl CoA (Fig. 5.3b). Assays of homocysteine and methylmalonic acid in plasma are used in there some laboratories as tests for B12 deficiency (see p. 69).
Figure 5.3 The biochemical reactions of vitamin B12 in humans. Ado B12, deoxyadenosylcobalamin; CoA, coenzyme A; THF, tetrahydrofolate.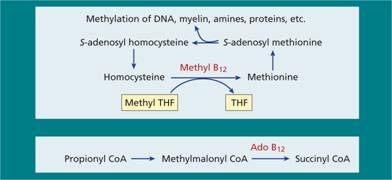
Folic (pteroylglutamic) acid is the parent compound of a large group of compounds, the folates, that are derived from it (Fig. 5.4). Humans are unable to synthesize the folate structure and thus require preformed folate as a vitamin.
Figure 5.4 The structure of folic (pteroylglutamic) acid. Dietary folates may contain: (a) additional hydrogen atoms at positions 7 and 8 (dihydrofolate) or 5, 6, 7 and 8 (tetrahydrofolate); (b) a formyl group at N5 or N10, a methyl group at N5 or other 1-carbon groups; and (c) additional glutamate moiety attached to the γ-carboxyl group of the glutamate moiety.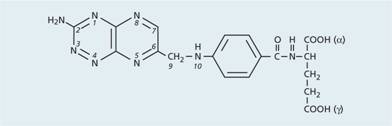
Absorption, transport and function
Dietary folates are converted to methyl THF (which, like folic acid, contains only one glutamate moiety) during absorption through the upper small intestine. Once inside the cell they are converted to folate polyglutamates (Fig. 5.5). Folate binding proteins are present on cell surfaces including the enterocyte and facilitate entry of reduced folates into cells. There is no specific plasma protein that enhances cellular folate uptake.
Figure 5.5 The biochemical basis of megaloblastic anaemia caused by vitamin B12 or folate deficiency. Folate is required in one of its coenzyme forms, 5, 10-methylene tetrahydrofolate (THF) polyglutamate, in the synthesis of thymidine monophosphate from its precursor deoxyuridine monophosphate. Vitamin B12 is needed to convert methyl THF, which enters the cells from plasma, to THF, from which polyglutamate forms of folate are synthesized. Dietary folates are all converted to methyl THF (a monoglutamate) by the small intestine. A, adenine; C, cytosine; d, deoxyribose; DHF, dihydrofolate; DP, diphosphate; G, guanine; MP, monophosphate; T, thymine; TP, triphosphate; U, uracil.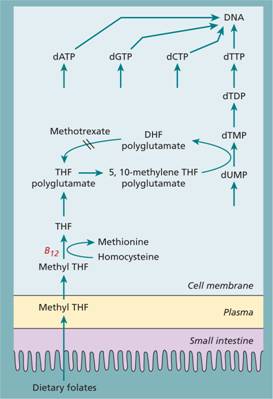
Folates are needed in a variety of biochemical reactions in the body involving single carbon unit transfer, in amino acid interconversions (e.g. homocysteine conversion to methionine) (Figs 5.3, 5.5) and serine to glycine or in synthesis of purine precursors of DNA.
Biochemical basis for megaloblastic anaemia (Fig. 5.5)
DNA is formed by polymerization of the four deoxyribonucleoside triphosphates. Folate deficiency is thought to cause megaloblastic anaemia by inhibiting thymidylate synthesis, a rate-limiting step in DNA synthesis in which thymidine monophosphate (dTMP) is synthesized. This reaction needs 5, 10-methylene THF polyglutamate as coenzyme.
All body cells, including those of the bone marrow, receive folate from plasma as methyl THF. B12 is needed in the conversion of this methyl THF to THF, a reaction in which homocysteine is methylated to methionine. THF (but not methyl THF) is a substrate for folate polyglutamate synthesis inside cells. The folate polyglutamates act as intracellular folate coenzymes, including 5, 10-methylene THF polyglutamate, the coenzyme form of folate involved in the synthesis of dTMP and of dTTP (Fig. 5.5). Lack of B12 prevents the demethylation of methyl THF, thus depriving cells of THF of 5, 10-methylene THF polyglutamate and so of dTMP and dTTP (Fig. 5.5).
Other congenital or acquired causes of megaloblastic anaemia (e.g. antimetabolite drug therapy) inhibit purine or pyrimidine synthesis at one or other step. The result is a reduced supply of one or other of the four precursors needed for DNA synthesis.
Folate reduction
During the synthesis of dTMP, the folate polyglutamate coenzyme becomes oxidized from the THF state to dihydrofolate (DHF) (Fig. 5.5). Regeneration of active THF requires the enzyme DHF reductase. Inhibitors of this enzyme (e.g. methotrexate) threesome fore inhibit all folate coenzyme reactions, and so DNA synthesis (Fig. 5.5). Methotrexate is a useful drug, mainly in the treatment of malignant or inflammatory disease (e.g. of the skin) with excessive cell turnover. The weaker antagonist, pyrimethamine, is used primarily against malaria. Trimethoprim, active against bacterial DHF reductase but only very weakly against the human enzyme, is used alone or in combination with a sulphonamide, as co-trimoxazole. Toxicity caused by methotrexate or pyrimethamine is reversed by giving the reduced folate, folinic acid (5-formyl THF).
In Western countries, severe deficiency is usually caused by (Addisonian) pernicious anaemia (Table 5.3). Less commonly, it may be caused by veganism in which the diet lacks B 12 (usually in Hindu Indians), gastrectomy or small intestinal lesions. Mild degrees of B12 deficiency have been reported in the elderly and ascribed to poor diet and malabsorption of food B12. There is no syndrome of B12 deficiency as a result of increased utilization or loss of the vitamin. The deficiency takes at least 2 years to develop (i.e. the time needed for body stores to deplete at the rate of 1–2μg/day) when there is severe malabsorption of B12 from the diet. Nitrous oxide, however, may rapidly inactivate body B12 (see p. 71).
Table 5.3 Causes of severe vitamin B12 deficiency.
| Nutritional |
| Especially vegans |
| Malabsorption |
| Gastric causes |
| Pernicious anaemia |
| Congenital lack or abnormality of intrinsic factor Total or partial gastrectomy |
| Intestinal causes |
| Intestinal stagnant loop syndrome – jejunal diverticulosis, blind-loop, stricture, etc. |
| Chronic tropical sprue |
| Ileal resection and Crohn’s disease |
| Congenital selective malabsorption with proteinuria (autosomal recessive megaloblastic anaemia) |
| Fish tapeworm |
Causes of mild vitamin B12 deficiency; other causes of malabsorption of vitamin B12 (e.g. malabsorption of food B12, in the elderly, atrophic gastritis, severe pancreatitis, gluten-induced enteropathy, HIV infection or therapy with metformin): These conditions do not usually lead to vitamin B12 deficiency sufficient to cause anaemia or neuropathy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


