AD, autosomal dominant; AR, autosomal recessive; RE, reticuloendothelial.
The tests that can be performed to assess iron overload are listed in Table 4.3. Tests may also be carried out to determine the degree of organ damage caused by iron (Table 4.3). The serum ferritin is the most widely used test and this and the percentage saturation of transferrin (iron-binding capacity) are useful screening tests for iron overload and for monitoring its treatment. Liver biopsy with staining for iron and chemical analysis of iron content is useful for assessing both parenchymal iron (hepatic cells) and reticuloendothelial iron in Kupffer cells. Magnetic resonance imaging (MRI), particularly the T2* technique, is the best non-invasive guide to liver and cardiac iron.
Table 4.3 Assessment of iron overload.
| Assessment of iron stores | |
| Serum ferritin | |
| Serum iron and percentage saturation of transferrin (iron-binding capacity) | |
| Serum non-transferrin bound iron | |
| Bone marrow biopsy (Perls’ stain) for reticuloendothelial stores | |
| > Liver biopsy (parenchymal and reticuloendothelial stores) | |
| Liver CT scan or MRI | |
| Cardiac MRI (T2* technique) | |
| Deferoxamine or deferiprone urine iron excretion test (chelatable iron) | |
| Repeated phlebotomy until iron deficiency occurs | |
| Assessment of tissue damage caused by iron overload | |
| Cardiac | Clinical; chest X-ray; ECG; 24-hour monitor; echocardiography; radionuclide (MUGA) scan to check left ventricular ejection fraction at rest and with stress |
| Liver | Liver function tests; liver biopsy; CT scan or MRI |
| Endocrine | Clinical examination (growth and sexual development); glucose tolerance test; pituitary gonadotrophin release tests; thyroid, parathyroid, gonadal, adrenal function, growth hormone assays; radiology for bone age; isotopic bone density study |
CT, computed tomography; ECG, electrocardiography; MRI, magnetic resonance imaging; MUGA, multiple gated acquisition.
Hereditary (genetic, primary) haemochromatosis
This is a group of diseases in which there is excessive absorption of iron from the gastrointestinal tract leading to iron overload of the parenchymal cells of the liver (Fig. 4.1), of the endocrine organs and, in severe cases, of the heart.
Figure 4.1 Liver biopsy. Iron loading of hepatic parenchymal cells (Perls’ stain). (Courtesy of Professor A.P. Dhillon)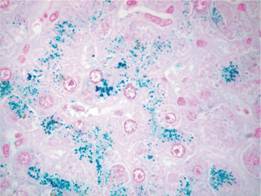
The most common gene involved is HFE and most patients are homozygous for a missense mutation (845 G to A) which leads to insertion of a tyrosine residue rather than cysteine in the mature protein (C282Y).This allele has a prevalence of approximately 1 in 300 within the white North European population. The HFE gene is situated close to the major histocompatibility complex (MHC) locus on chromosome 6. The abnormal allele is associated with HLA-A3 and-B8. Only a small proportion of those homozygous for the mutation present with clinical features of the disease and these usually show a serum ferritin > 1000 μg/L. A second mutation resulting in a histidine to aspartic acid substitution H63D is found with the C282Y mutation in approximately 5% of patients but homozygotes for the H63D mutation do not have the disease.
Serum hepcidin levels are low in patients with mutated HFE because HFE is involved in hepcidin synthesis or secretion (see Fig. 3.4). Low serum hepcidin levels lead to high levels of ferroportin on the basolateral surface of the duodenal enterocyte and so lead to increased iron absorption and increased release of iron from macrophages.
The consequent iron overload damages parenchymal cells and patients may present in adult life with hepatic disease, endocrine disturbances such as diabetes mellitus or impotence, melanin skin pigmentation (Fig. 4.2) and arthropathy (resulting from pyrophosphate deposition). In some severe cases there is cardiac failure or arrhythmia. Diagnosis is suspected by increased serum iron, increased serum transferrin saturation and ferritin. It is confirmed by testing for the HFE mutation. Liver biopsy may quantify the degree of iron overload and assess liver damage. MRI can also be used to measure liver and cardiac iron.
Figure 4.2 Melanin skin pigmentation. The right hand is of a teenager with iron overload caused by thalassaemia major. The left hand is of her mother who has normal iron status.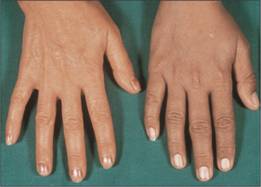
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


