Anaemia of chronic disorders
Many of the anaemias seen in clinical practice occur in patients with systemic disorders and are the result of a number of contributing factors. The anaemia of chronic disorders (also discussed on p. 46) is of central importance and occurs in patients with a variety of chronic inflammatory and malignant diseases (Table 28.1). Usually, both the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are raised. It may be complicated by additional haematological changes caused by the disease. The serum iron and total iron binding capacity (transferrin) are both low; serum ferritin can benormal or raised. The characteristic features aredescribed in Chapter 3
Table 28.1 Causes of anaemia of chronic disorders.
| Chronic inflammatory diseases |
| Infectious (e.g. pulmonary abscess, tuberculosis, osteomyelitis, pneumonia, bacterial endocarditis) |
| Non-infectious (e.g. rheumatoid arthritis, systemic lupus erythematosus and other connective tissue diseases, sarcoid, Crohn’s disease, cirrhosis) |
| Malignant disease (e.g. carcinoma, lymphoma, sarcoma, myeloma) |
The pathogenesis of this anaemia appears to berelated to the decreased release of iron from macrophagesto plasma and so to erythroblasts, caused byhepcidin, reduced red cell lifespan and an inadequate erythropoietin response to anaemia. The plasma levels of various cytokines, especially interleukin-1 (IL-1), IL-6 and tumour necrosis factor (TNF) are raised and reduce erythropoietin secretion. The anaemia is corrected by the successful treatment of the underlying disease. It does not respond to iron therapy despite the low serum iron. Responses to recombinant erythropoietin therapy may be obtained (e.g. in rheumatoid arthritis or cancer). In many conditions the anaemia is complicated by anaemia from other causes (e.g. iron or folate deficiency, renal failure, bone marrow infiltration, hypersplenism or endocrine abnormality).
Malignant diseases (other than primary bone marrow diseases)
Anaemia
Contributing factors include anaemia of chronic disorders, blood loss and iron deficiency, marrow infiltration (Fig. 28.1) often associated with a leucoerythroblastic blood film (see p. 118), folate deficiency, haemolysis and marrow suppression from radiotherapy or chemotherapy (Table 28.2).
Figure 28.1 Metastatic carcinoma in bone marrow aspirates: (a) breast; (b) stomach; (c) colon; bone marrow trephine biopsies: (d) prostate; (e) stomach; (f) kidney.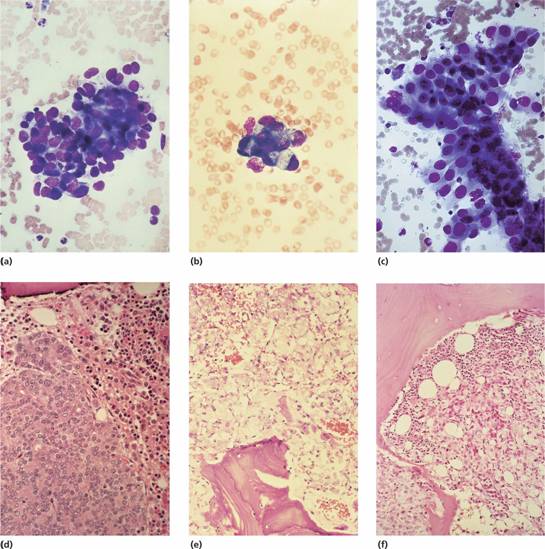
Table 28.2 Haematological abnormalities in malignant disease.
| Haematological abnormality | Tumour or treatment associated |
| Pancytopenia | |
| Marrow hypoplasia | Chemotherapy, radiotherapy |
| Myelodysplasia | Chemotherapy, radiotherapy |
| Leucoerythroblastic | Metastases in marrow |
| Megaloblastic | Folate deficiency |
| B12 deficiency (carcinoma of stomach) | |
| Red cells | |
| Anaemia of chronic disorders | Most forms |
| Iron deficiency anaemia | Especially gastrointestinal, uterine |
| Pure red cell aplasia | Thymoma |
| Immune haemolytic anaemia | Lymphoma, ovary, other tumours |
| Microangiopathic haemolytic anaemia | Mucin-secreting carcinoma |
| Polycythaemia | Kidney, liver, cerebellum, uterus |
| White cells | |
| Neutrophil leucocytosis | Most forms |
| Leukaemoid reaction | Disseminated tumours, those with necrosis |
| Eosinophilia | Hodgkin lymphoma, others |
| Monocytosis | Various tumours |
| Platelets and coagulation | |
| Thrombocytosis | Gastrointestinal tumours with bleeding, others |
| Disseminated intravascular coagulation | Mucin-secreting carcinoma, prostate |
| Activation of fibrinolysis | Prostate |
| Acquired inhibitors of coagulation | Most forms |
| Paraprotein interfering with platelet function | Lymphomas, myeloma |
| Tumour cell procoagulants – tissue factor and cancer procoagulant (activates factor X) | Especially ovarian, pancreas, brain, colon |
Microangiopathic haemolytic anaemia (see p. 85) occurs with mucin-secreting adenocarcinoma (Fig. 28.2), particularly of the stomach, lung and breast. Less common forms of anaemia with malignant disease include autoimmune haemolytic anaemia with malignant lymphoma and rarely with other tumours; primary red cell aplasia with thymoma or lymphoma; and myelodysplastic syndromes secondary to chemotherapy. There is also an association of pernicious anaemia with carcinoma of the stomach.
Figure 28.2 Peripheral blood film in metastatic mucin-secreting adenocarcinoma of the stomach showing red cell polychromasia and fragmentation and thrombocytopenia. The patient had disseminated intravascular coagulation.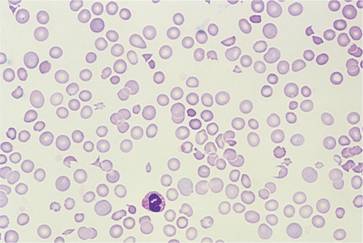
The anaemia of malignant disease may respond partly to erythropoietin but this may accelerate tumour growth. Folic acid should only be given if there is definite megaloblastic anaemia caused by the deficiency; it might ‘feed’ the tumour.
Polycythaemia
Secondary polycythaemia is occasionally associated with renal, hepatic, cerebellar and uterine tumours (see p. 208).
White cell changes
Leukaemoid reactions (see p. 117) may occur with tumours showing widespread necrosis and inflammation. Hodgkin lymphoma is associated with a variety of white cell abnormalities including eosinophilia, monocytosis and leucopenia. In non-Hodgkin lymphoma, malignant cells may circulate in the blood (see p. 260).
Platelet and blood coagulation abnormalities
Patients with malignant disease may show either thrombocytosis or thrombocytopenia. Disseminated tumours, particularly mucin-secreting adenocarcinomas, are associated with disseminated intravascular coagulation (DIC; see p. 355) and generalized haemostatic failure. Activation of fibrinolysis occurs in some patients with carcinoma of the prostate. Occasional patients with malignant disease have spontaneous bruising or bleeding caused by an acquired inhibitor of one or other coagulation factor, most frequently factor VIII, or to a paraprotein interfering with platelet function.
Cancer patients have a high incidence (estimated at 15%) of venous thromboembolism. This is increased by surgery and some drugs (e.g. thalidomide). It is most common in ovarian, brain, pancreatic and colon cancers. It may be difficult to manage with oral anticoagulation because of bleeding, interruptions with chemotherapy and thrombocytopenia, anorexia or vomiting. Liver disease and drug interactions can cause further complications so daily low molecular weight heparin injections may be preferable to oral anticoagulants.
Rheumatoid arthritis (and other connective tissue disorders)
In patients with rheumatoid arthritis, the anaemia of chronic disorders is proportional to the severity of the disease. It is complicated in some patients by iron deficiency caused by gastrointestinal bleeding related to therapy with salicylates, other non-steroidal anti-inflammatory agents or corticosteroids. Bleeding into inflamed joints may also be a factor. Marrow hypoplasia may follow therapy with gold. In Felty’s syndrome, splenomegaly is associated with neutropenia (Fig. 28.3). Anaemia and thrombocytopenia may also be present.
Figure 28.3 Felty’s syndrome: (a) the typical deformities of rheumatoid arthritis of the hand; and (b) splenomegaly.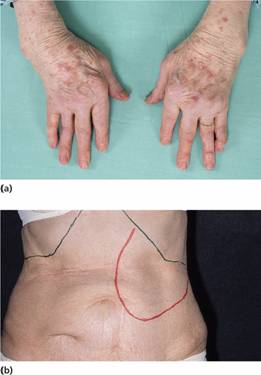
In systemic lupus erythematosus (SLE) there may be anaemia of chronic disorders and 50% of patients are leucopenic with reduced neutrophil and lymphocyte counts often associated with circulating immune complexes. Renal impairment and drug-induced gastrointestinal blood loss also contribute to the anaemia. Autoimmune haemolytic anaemia (typically with immunoglobulin G (IgG) and the C3 component of complement on the surface of the red cells) occurs in 5% of patients and may be the presenting feature of the syndrome. There may be autoimmune thrombocytopenia in 5% of patients. The lupus anticoagulant is described on p. 369. This circulating anticardiolipin interferes with blood coagulation by altering the binding of coagulation factors to platelet phospholipid and predisposes to both arterial and venous thrombosis and recurrent abortions. The antibody may be responsible for a false positive Wassermann reaction. Tests for antinuclear factor and anti-DNA antibodies are usually positive.
Patients with temporal arteritis and polymyalgia rheumatica have a markedly elevated ESR, pronounced red cell rouleaux in the blood film and a polyclonal immunoglobulin response. These and other collagen vascular disorders are associated with anaemia of chronic disorders.
Anaemia
A normochromic anaemia is present in most patients with chronic renal failure. Generally, there is a 2g/dL fall in haemoglobin level for every 10 mmol/L rise in blood urea. There is impaired red cell production as a result of defective erythropoietin secretion (see Fig. 2.5). Uraemic serum has also been shown to contain factors that inhibit proliferation of erythroid progenitors but, in view of the excellent response to erythropoietin in most patients, the clinical relevance of these is doubtful. Variable shortening of red cell lifespan occurs and in severe uraemia the red cells show abnormalities including spicules (spurs) and ‘burr’ cells (Fig. 28.4). Increased red cell 2,3-diphosphoglycerate (2,3-DPG) levels in response to the anaemia and hyperphosphataemia result in decreased oxygen affinity and a shift of the haemoglobin oxygen dissociation curve to the right (see p. 21), which is augmented by uraemic acidosis. The patient’s symptoms are therefore relatively mild for the degree of anaemia.
Figure 28.4 Peripheral blood film in chronic renal failure showing red cell acanthocytosis and numerous ‘burr’ cells.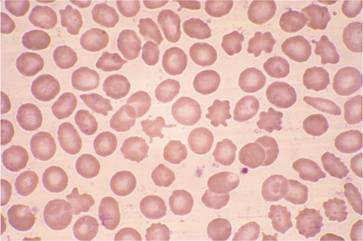
Other factors may complicate the anaemia of chronic renal failure (Table 28.3): the anaemia of chronic disorders, iron deficiency from blood loss during dialysis or caused by bleeding because of defective platelet function, and folate deficiency in some chronic dialysis patients. Aluminium excess in patients on chronic dialysis also inhibits erythropoiesis. Patients with polycystic kidneys usually have retained erythropoietin production and may have less severe anaemia for the degree of renal failure.
Table 28.3 Haematological abnormalities in renal failure.
| Anaemia |
| Reduced erythropoietin production |
| Aluminium excess in dialysis patients |
| Anaemia of chronic disorders |
| Iron deficiency blood loss (e.g. dialysis, venesection, defective platelet function) |
| Folate deficiency chronic haemodialysis without replacement therapy |
| Abnormal platelet function |
| Thrombocytopenia |
| Immune complex-mediated (e.g. systemic lupus erythematosus, polyarteritis nodosa) |
| Some cases of acute nephritis and following allograft |
| Haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura |
| Thrombosis |
| Some cases of the nephrotic syndrome |
| Polycythaemia |



