Site of haemopoiesis
In the first few weeks of gestation the yolk sac is the main site of haemopoiesis. However, definitive haemopoiesis derives from a population of stem cells first observed on the dorsal aorta termed the AGM (aorta-gonads-mesonephros) region. These common precursors of endothelial and haemopoietic cells (haemangioblasts) are believed to seed the liver, spleen and bone marrow and from 6 weeks until 6–7 months of fetal life the liver and spleen are the major haemopoietic organs and continue to produce blood cells until about 2 weeks after birth (Table 1.1; see Fig. 7.1b). The bone marrow is the most important site from 6 to 7 months of fetal life. During normal childhood and adult life the marrow is the only source of new blood cells. The developing cells are situated outside the bone marrow sinuses; mature cells are released into the sinus spaces, the marrow microcirculation and so into the general circulation.
Table 1.1 Sites of haemopoiesis.
| Fetus | 0–2 months (yolk sac) |
| 2–7 months (liver, spleen) | |
| 5–9 months (bone marrow) | |
| Infants | Bone marrow (practically all bones) |
| Adults | Vertebrae, ribs, sternum, skull, sacrum and pelvis, proximal ends of femur |
In infancy all the bone marrow is haemopoietic but during childhood there is progressive fatty replacement of marrow throughout the long bones so that in adult life haemopoietic marrow is confined to the central skeleton and proximal ends of the femurs and humeri (Table 1.1 ). Even in these haemopoietic areas, approximately 50% of the marrow consists of fat (Fig. 1.1). The remaining fatty marrow is capable of reversion to haemopoiesis and in many diseases there is also expansion of haemopoiesis down the long bones. Moreover, the liver and spleen can resume their fetal haemopoietic role (‘extramedullary haemopoiesis’).
Figure 1.1 A normal bone marrow trephine biopsy (posterior iliac crest). Haematoxylin and eosin stain; approximately 50% of the intertrabecular tissue is haemopoietic tissue and 50% is fat.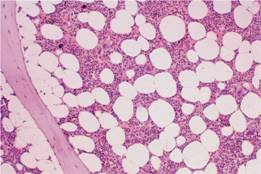
Haemopoietic stem and progenitor cells
Haemopoiesis starts with a pluripotential stem cell that can self-renew but also give rise to the separate cell lineages. These cells are able to repopulate a bone marrow from which all stem cells have been eliminated by lethal irradiation or chemotherapy. This haemopoietic stem cell is rare, perhaps 1 in every 20 million nucleated cells in bone marrow. Although its exact phenotype is unknown, on immunological testing it is CD34+ CD38− and negative for lineage markers (Lin−) and has the appearance of a small or medium-sized lymphocyte (see Fig. 23.3). The cells reside in specialized ‘niches’. Cell differentiation occurs from the stem cell via committed haemopoietic progenitors which are restricted in their developmental potential (Fig. 1.2). The existence of the separate progenitor cells can be demonstrated by in vitro culture techniques. Very early progenitors are assayed by culture on bone marrow stroma as long-term culture initiating cells whereas late progenitors are generally assayed in semi-solid media. An example is the earliest detectable mixed myeloid precursor which gives rise to granulocytes, erythrocytes, monocytes and megakaryocytes and is termed CFU (colony-forming unit)-GEMM (Fig. 1.2). The bone marrow is also the primary site of origin of lymphocytes (see Chapter 9) which differentiate from a common lymphoid precursor.
Figure 1.2 Diagrammatic representation of the bone marrow pluripotent stem cell and the cell lines that arise from it. Various progenitor cells can be identified by culture in semi-solid medium by the type of colony they form. It is possible that an erythroid/megakaryocytic progenitor may be formed before the common lymphoid progenitor diverges from the mixed granulocytic/monocyte/eosinophil myeloid progenitor. Baso, basophil; BFU, burst – forming unit; CFU, colony-forming unit; E, erythroid; Eo, eosinophil; GEMM, granulocyte, erythroid, monocyte and megakaryocyte; GM, granulocyte, monocyte; Meg, megakaryocyte; NK, natural killer.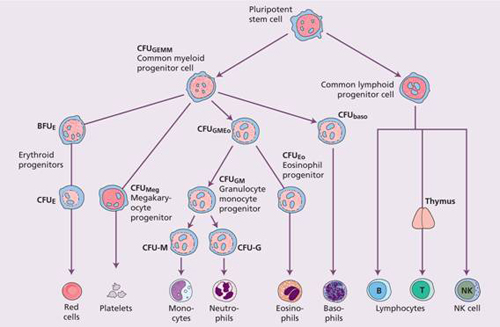
The stem cell has the capability for self-renewal (Fig. 1.3) so that marrow cellularity remains constant in a normal healthy steady state. There is considerable amplification in the system: one stem cell is capable of producing about 106 mature blood cells after 20 cell divisions (Fig. 1.3). The precursor cells are, however, capable of responding to haemopoietic growth factors with increased production of one or other cell line when the need arises. The development of the mature cells (red cells, granulocytes, monocytes, megakaryocytes and lymphocytes) is considered further in other sections of this book.
Figure 1.3 (a) Bone marrow cells are increasingly differentiated and lose the capacity for self – renewal as they mature. (b) A single stem cell gives rise, after multiple cell divisions (shown by vertical lines), to > 106 mature cells.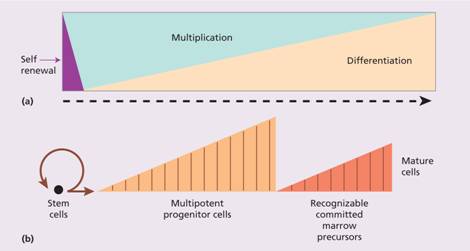
The bone marrow forms a suitable environment for stem cell survival, self-renewal and formation of differentiated progenitor cells. It is composed of stromal cells and a microvascular network (Fig. 1.4). The stromal cells include adipocytes, fibroblasts, osteoblasts, endothelial cells and macrophages and they secrete extracellular molecules such as collagen, glycoproteins (fibronectin and thrombospondin) and glycosaminoglycans (hyaluronic acid and chondroitin derivatives) to form an extracellular matrix. In addition, stromal cells secrete several growth factors necessary for stem cell survival.
Figure 1.4 Haemopoiesis occurs in a suitable microenvironment (‘niche’) provided by a stromal matrix on which stem cells grow and divide. There are specific recognition and adhesion sites (see p. 13); extracellular glycoproteins and other compounds are involved in the binding.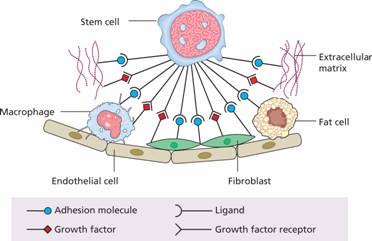
Mesenchymal stem cells , also called multipotent mesenchymal stromal cells or adherent stromal cells, are critical in stromal cell formation. Together with osteoblasts they form niches and provide the growth factors, adhesion molecules and cytokines which support stem cells, e.g. the protein jagged, on stromal cells binds to a receptor NOTCH1 on stem cells which then becomes a transcription factor involved in the cell cycle.
Stem cells are able to traffic around the body and are found in peripheral blood in low numbers. In order to exit the bone marrow, cells must cross the blood vessel endothelium and this process of mobilization is enhanced by administration of growth factors such as granulocyte colony – stimulating factor (G-CSF) (see p. 6 ). The reverse process of stem cell homing appears to depend on a chemokine gradient in which the stromal-derived factor 1 (SDF-1) is critical. Several critical interactions maintain stem cell viability and production in the stroma including stem cell factor (SCF) and jagged proteins expressed on stroma and their respective receptors KIT and NOTCH expressed on stem cell.
Stem cells are present in many different organs. These are pluripotent and can generate various types of tissue, e.g. epithelial cells, nerve cells (Fig. 1.5). Studies in patients and animals who have received haemopoietic stem cell transplants (see Chapter 23) have suggested that donor haemopoietic cells may contribute to tissues such as neurons, liver and muscle. The contribution of adult donor haemopoietic cells to non-haemopoietic tissues is at most very small. The persistence of pluripotential stem cells in postnatal life, the presence of mesenchymal stem cells in bone marrow and fusion of transplanted cells with host cells have all been proposed to explain many of the findings suggesting stem cell ‘plasticity’.
Figure 1.5 (a) Cells from the inner cell mass of the blastocyst in the early embryo are able to generate all the tissues of the body and are known as totipotent. (b) Specialized adult stem cells of the bone marrow, nervous tissue, epithelial and other tissues give rise to differentiated cells of the same tissue.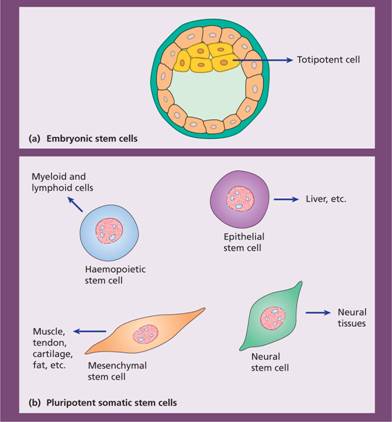
The regulation of haemopoiesis
Haemopoiesis starts with stem cell division in which one cell replaces the stem cell (self-renewal) and the other is committed to differentiation. These early committed progenitors express low levels of transcription factors that may commit them to discrete cell lineages. Which cell lineage is selected for differentiation may depend both on chance and on the external signals received by progenitor cells. Several transcription factors (see p. 13 ) regulate survival of stem cells (e.g. SCL, GATA-2, NOTCH-1) whereas others are involved in differentiation along the major cell lineages. For instance, PU.1 and the CEBP family commit cells to the myeloid lineage whereas GATA-1 and FOG-1 have an essential roles in erythropoietic and megakaryocytic differentiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


