Soft Tissue and Bone Sarcomas
SOFT TISSUE SARCOMAS
Soft tissue sarcomas (STS) are uncommon malignancies, arising in about 11,410 persons in the United States each year and accounting for 4390 deaths, mostly due to either locoregional recurrence or distant metastasis (1–4). Although malignant tumors of soft tissue are scarce, benign tumors such as lipomas are 100 times more common. STS occur at any age with a median age of around 50-years old, and are equally common in men and women.
STS constitute a highly heterogeneous group of tumors with respect to anatomical distribution, histologic subtype, and clinical behavior (1). STS occur throughout the body, but nearly one-half occur in the extremities, with about one-third occurring in the lower extremity and 15% occurring in the upper extremity. Another one-third of STS occur in the abdomen, and these are equally divided among intra-abdominal visceral sarcomas (primarily gastrointestinal stromal tumors and leiomyosarcomas) and retroperitoneal sarcomas. Other anatomic sites include the head/neck, trunk, and other miscellaneous sites (e.g., heart).
STS are malignant tumors which arise from the mesodermal tissues (e.g., fat, muscle, connective tissue, and vessels) excluding bone and cartilage. In addition, malignant tumors of peripheral nerve sheaths are usually included despite being ectodermal in origin. There are over 50 different histologic subtypes of STS with the most common being liposarcoma, leiomyosarcoma, fibrosarcoma, and synovial sarcoma. Malignant fibrous histiocytoma was historically the most common subtype but the majority of these are now classified as other subytpes including undifferentiated pleomorphic sarcomas. All suspected STS cases should be reviewed by a pathologist experienced in sarcomas given that about 10% of cases originally designated as STS are in fact not STS and about 20% are initially assigned the incorrect histologic subtype (5). While each histologic subtype may have certain specific clinical behaviors, all STS can generally be categorized into low-, intermediate-, and high-grade tumors. Low-grade tumors grow more slowly, can locally recur after resection, but have a low risk of distant metastases (about 5%). High-grade tumors tend to grow more rapidly, can recur locally, and have the added risk of distant metastasis that can approach 50% for large tumors greater than 5–10 cm in largest dimension.
The treatment of STS has advanced significantly over the past few decades. In particular, evidence has accumulated that in addition to surgery, there are important roles for radiation therapy and chemotherapy in the management of some STS patients. Optimal results from more conservative local treatment strategies require a multidisciplinary approach to the overall management of these patients. The team should include not only an experienced and specialized surgeon, but also a radiation oncologist, medical oncologist, pathologist, and diagnostic radiologist expert in the disease. Additional specialists who may be important in the care of these patients include plastic/reconstructive surgeons, physiatrists working with physical and occupational therapists, psychiatrists, psychologists, and social workers. For this relatively uncommon solid tumor that occurs throughout the body and has over 50 histologic subtypes, evaluation and treatment is best done at a tertiary referral center.
 ETIOLOGY
ETIOLOGY
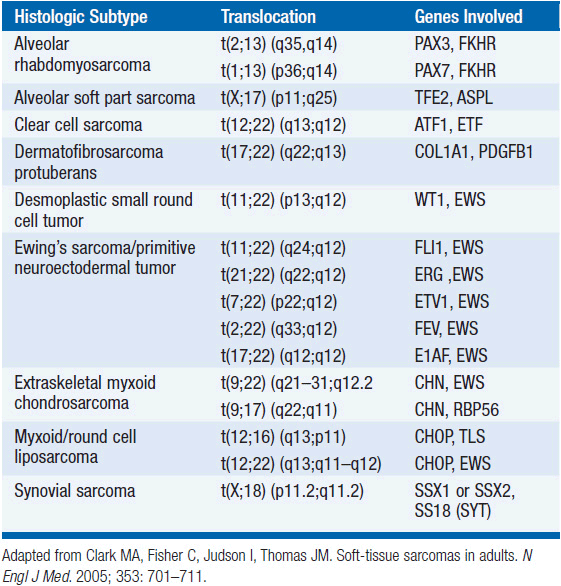
The vast majority of STS occur as sporadic tumors in patients with no identified genetic or environmental risk factors. However, certain genetic syndromes are associated with an increased risk of developing sarcomas including neurofibromatosis 1 (NF1, von Recklinghausen’s disease), hereditary retinoblastoma, and Li-Fraumeni syndrome. Specific genetic abnormalities, evidenced by nonrandom chromosomal aberrations, are well established in certain STS histologic subtypes, and are often utilized in the definitive diagnosis (Table 61-1).
Radiation is recognized as capable of inducing sarcomas of bone and soft tissue. The frequency increases with radiation dose and with the postradiation observation period. Chemotherapeutic agents are likewise associated with risks of sarcoma induction. STS (primarily lymphangiosarcomas) may be observed following massive and quite protracted edema after axillary lymph-adenectomy (Stewart–Treves syndrome). Trauma is rarely a factor in the development of these tumors with the possible exception of desmoid tumors.
 STAGING
STAGING
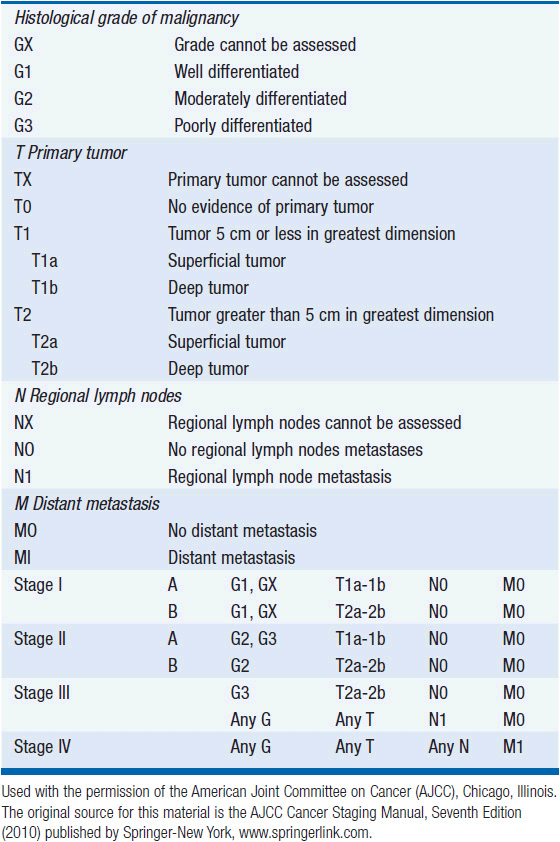
The Task Force on STS of the American Joint Committee on Cancer (AJCC) Staging and End Result Reporting has established a staging system for STS which is an extension of the TNM system to include G for histological grade (Table 61-2). Grade, size, depth, and presence of nodal or distant metastases are the determinants of stage. Of these, grade is particularly important in staging sarcomas. Some institutions will assign grades 1–3, where grade 1 lesions are considered low grade with minimal metastatic potential and the intermediate grade 2 and high grade 3 lesions are considered high grade and capable of metastatic disease. Other institutions use a 2- or 4-tiered system.
TABLE 61-2 AJCC STAGING SYSTEM FOR SOFT TISSUE SARCOMAS
EXTREMITY STS
 CLINICAL EVALUATION
CLINICAL EVALUATION
The most frequent initial complaint is that of a painless, enlarging mass for a few weeks to several months. Occasionally, pain or tenderness precedes the detection of a mass. With progressive growth of the tumor, symptoms appear which are usually secondary to infiltration of or pressure on adjacent structures. Interestingly, some high-grade sarcomas in the foot or ankle may have been noticed initially several years prior to diagnosis. One should obtain a complete history and physical examination, with particular attention paid to the region of the primary lesion: definition of size, site of origin (superficial or deep, attached to or fixed to deep structures), involvement or discoloration of overlying skin, functional status of vessels and nerves, mass effect on adjacent organs and joints, and presence of distal edema. Laboratory studies need not go beyond a complete blood count and chemistry panel. There are no tumors markers for STS.
For the primary site, the radiographic evaluation should include a CT scan or MRI. The most useful radiologic study to evaluate an extremity or trunk primary site is the MRI, but CT scans can provide supplemental information. A chest CT should be obtained for high-grade tumors to evaluate for lung metastases. A chest x-ray may be adequate for low-grade tumors. The role of PET scans has yet to be defined, but many primary and metastatic tumors may show increased FDG uptake especially as the grade increases.
An adequate biopsy is required to determine a histologic diagnosis as to tumor type and grade and to determine an optimal treatment strategy. In the majority of cases, the diagnosis can be established by core needle biopsy. Superficial lesions which are readily palpable can be directly biopsied with ultrasound guidance (all lesions should be imaged prior to biopsy), but for tumors which are located at a depth which makes the lesion appear less well defined, a CT-directed approach is advocated. Open biopsies are done less commonly and should be reserved for the uncommon cases where core biopsy is not adequate. The incision for open biopsies should usually be oriented longitudinally such that it can be easily incorporated in the definitive resection. For tumors >3–5 cm in size, and depending on where they are located (i.e., hand or foot are exceptions), an excisional biopsy can sometimes be performed, and incisional biopsies can be performed for larger lesions. Care should be taken to minimize bleeding and contamination of surrounding tissues. Fine needle biopsy can best be employed to confirm metastatic or recurrent tumor when the primary diagnosis is already established.
 SURGERY AND RADIATION THERAPY
SURGERY AND RADIATION THERAPY
If STS are “shelled out” as is performed for benign tumors such as lipomas, the local recurrence will be up to 90%. Radical resection of tumors with a margin of normal tissue can decrease the local recurrence rate to 10%–30%. However, many extremity STS grow adjacent to major blood vessels and bones, and the standard operation for many STS up until the early 1980s was amputation. Rosenberg et al. at the National Cancer Institute (NCI) published a randomized trial of amputation versus limb-sparing surgery and radiation (both groups received chemotherapy) in 1982 and demonstrated equivalent overall survival with a local recurrence rate of 0% versus 15% (6). Currently limb-sparing surgery can be performed in over 90% of patients with extremity STS, and overall local recurrence rates are often less than 10%.
Several surgical principles should be followed when resecting STS. First, the preoperative imaging studies should be carefully examined to identify the full extent of tumor penetration as well as the relationship of the tumor to adjacent structures. Second, tumors should be resected with an adequate margin of normal tissue if this can be performed without severe morbidity. The distance is variable and probably can be reduced when high-quality margins such as fascia are present as the border. Tissues such as fat or muscle probably require 1–2 cm of normal tissue because of frequent infiltration of the tumor cells within these tissues. One can often accept a few millimeters of fascia margin but should be more concerned about a close margin of fat or muscle. Third, STS usually do not invade the periadventitial tissue of arteries or the periosteum of bone and can often be dissected along these planes. However, some STS actually arise in the vessels or nerves precluding salvage in terms of resection. Surgeons in general must use considerable judgment in resecting STS and careful discussion with bone and soft tissue radiologists prior to the procedure is important. Positive microscopic margins are very strongly associated with an increased risk of local recurrence, and one should strive for negative microscopic margins in all cases unless this would create major or unacceptable morbidity. In such cases one may rely on adjuvant radiation therapy in order to reduce major surgical morbidity, given radiation can usually be delivered in doses to the extremity that can eradicate microscopic residual disease.
Several studies have defined the essential role of radiation therapy in the local control of STS. Another NCI randomized trial published in 1998 comparing limb-spring surgery alone to surgery and external beam radiation (patients with high-grade tumors all received chemotherapy) demonstrated that radiation reduced local recurrence from 20%–33% to 0%–4% (7). The rate of distant recurrence was the same in both groups. Brachytherapy has also been used to deliver radiation. In a randomized trial of surgery alone versus surgery and brachytherapy, local recurrence for high-grade tumors was reduced from 30% to 5% with brachytherapy. At our institution, brachytherapy is often employed for patients who have a local recurrence after prior surgery and radiation, and this allows the delivery of additional radiation while minimizing morbidity. Brachytherapy or intraoperative radiation therapy can also be used at the time of initial surgery and radiation to deliver a focal boost to areas of close or positive surgical margins.
The order of radiation therapy in relation to surgery is a subject of debate between major sarcoma centers. One randomized trial by the Canadian NCI examined preoperative and postoperative radiation therapy and found no difference in local control (8). Complications were twice as high in the preoperative therapy group (35% vs 17%), but tissue fibrosis and other late complications were more frequent in the postoperative radiation group.
There are certain situations such as difficult anatomical location (e.g., pelvis, spine, or base of skull) or major medical comorbidities (e.g., cardiac dysfunction or metastatic lung cancer) in which a conservative surgical procedure may have much apparent risk and radiation alone is delivered. In one study from Memorial Sloan-Kettering, 25 patients were treated by radiotherapy alone, and local control was achieved in 14 of the 25 patients. To achieve a high probability of local control, higher doses than would be used for residual microscopic disease in the range of 70–75 Gy are essential. Among patients receiving ≥63 Gy, Kepka et al. reported local control in 72% of patients with tumors ≥5 cm in size, 42% for lesions >5 cm but ≤10 cm, and only 25% for lesions >10 cm (9). Because high doses are required for control of these unresected lesions, treatment techniques such as proton beam radiation, or even conventional radiation in an intensity-modulated fashion may be required to deliver dose and stay within the constraints of normal tissue tolerance.
Patients with large (>8–10 cm), deep, high-grade sarcomas present more difficult problems in terms of local control and are at significant risk of distant metastasis. Some groups have combined chemotherapy with surgery and radiation therapy strategies. Eilber and Morton have been proponents of a program, which has consisted of intra-arterial doxorubicin followed by rapid fraction hypofractionated radiation therapy (3.5 Gy/fraction, 28 Gy total) and subsequent local excision. Their data have shown local recurrence rates of <10% with survival rates of 74% in stage III tumors. Among these patients, there was a 5% amputation rate. They have since shown that it is not necessary to provide the doxorubicin by an intra-arterial route. Our institution has employed a regimen of preoperative mesna, Adriamycin, ifosfamide, and dacarbazine (MAID) chemotherapy interdigitated with radiation therapy (44 Gy) followed by postoperative MAID chemotherapy for patients with >8 cm, high-grade STS (10). Five-year local control was 92% and 5-year overall survival was 87%. In experimental protocols, hyperthermic isolated limb perfusion (HILP) with chemotherapeutic agents (e.g., tumor necrosis factor alpha, melphalan, and interferon gamma) has been used to control large tumors that would otherwise require amputation because of proximity to nerve or blood vessels.
 ADJUVANT CHEMOTHERAPY
ADJUVANT CHEMOTHERAPY
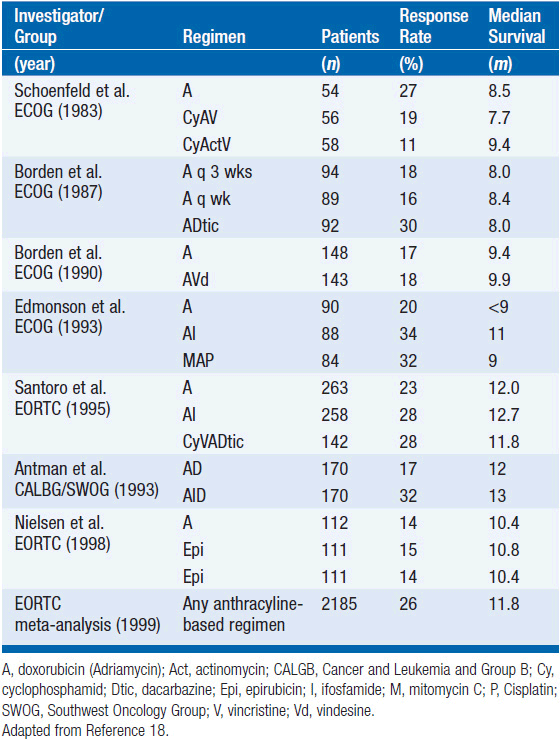
Although surgery and radiotherapy achieve control of the primary tumor in the majority of patients, many patients (especially those with large, high-grade tumors) develop and die of metastatic disease not evident at diagnosis. Doxorubicin and ifosfamide are the most active chemotherapy agents in metastatic STS. For doxorubicin, objective response rates between 20% and 40% have been reported. Several prospective studies using single-agent doxorubicin failed to show an improvement in disease-free or overall survival in patients receiving postoperative chemotherapy compared with surgery alone. The large EORTC study of adjuvant chemotherapy employed CyVADic consisting of cyclophosphamide, vincristine, doxorubicin, and DTIC found improved 7-year recurrence-free survival (56% vs 43%) but no significant difference in overall survival (63% vs 56%) (11). A meta-analysis of 14 randomized trials of doxorubicin-based adjuvant chemotherapy versus no chemotherapy in STS was performed in 1997 (12) and updated in 2008 with three additional studies using ifosfamide with doxorubicin (13). The adjuvant chemotherapy group had a statistically significant improved rate of distant and overall recurrence (OR was 0.67; 95% CI 0.56–0.82; P = 0.0001), distant recurrence-free survival (70% vs 60%, P = 0.003), but overall survival was only significantly improved in the studies that combined ifosfamide with doxorubicin (OR = 0.56; 95% CI, 0.36–0.85; P = 0.01). However, the toxicities associated with these chemotherapeutic agents dictate caution in generalizing adjuvant chemotherapy to all patients (indeed, different sarcoma centers in North America and Europe have varying degrees of enthusiasm for the use of adjuvant chemotherapy), which argues for and favor continuing enrollment of patients in clinical trials where available.
 RETROPERITONEAL STS
RETROPERITONEAL STS
Approximately 10%–15% of soft tissue sarcomas arise in the retroperitoneum. Tumors may be identified on imaging studies for unrelated complaints, or patients may also present with a palpable abdominal mass or with symptoms such as abdominal pain or lower extremity neurologic symptoms. Since the retroperitoneum can accommodate large tumors without symptoms, the average size of tumors in large series is often greater than 10 cm. Upon histologic examination, about two-thirds of tumors are either liposarcomas or leiomyosarcomas, with the remaining tumors distributed among a large variety of other histologic subtypes. Retroperitoneal liposarcomas are further subclassified into well-differentiated/dedifferentiated, myxoid/round cell, and pleomorphic subtypes. Most unifocal tumors in the retroperitoneum that do not arise from adjacent organs will be either benign soft tissue tumors (e.g., schwannomas, paraganglioneuroma, or neurofibroma) or sarcomas. Other malignancies in the differential diagnosis include primary germ cell tumor, metastatic testicular cancer, and lymphoma. Following a careful history and physical examination, radiologic assessment of these tumors is usually performed with an abdominal and pelvic CT scan. Liposarcomas often have a characteristic appearance with large areas of abnormal-appearing fat (well-differentiated liposarcoma) sometimes containing higher-density nodules (dedifferentiated liposarcoma). Patients with high-grade tumors should have a chest CT to evaluate for lung metastases.
The primary treatment for the local control of these tumors is surgical resection (14). The optimal goal of surgical resection is complete gross resection with microscopically negative margins, but this can be difficult to accomplish, and complete gross resection rates in large series are reported to be around 60%. In about three-quarters of cases, complete gross resection requires removal of adjacent viscera. The goal of obtaining negative microscopic margins for large retroperitoneal tumors is frequently not achieved. These tumors are surrounded by a pseudocapsule that often contains microscopic disease, and dissection with a normal tissue margin away from the pseudocapsule is difficult, especially along the posterior aspect of the tumor where it abuts the retroperitoneal fat and musculature. Controversy exists as to the optimal role of radiation therapy for local control of retroperitoneal sarcomas. Those who advocate radiation therapy usually prefer that radiation be delivered preoperatively. With the tumor still in place, normal organs are pushed away from the radiation field, the margin around the tumor at risk of local recurrence is more clearly defined, and the effective radiation dose required to control microscopic disease is likely lower.
In the extremity, the local control of sarcomas treated with total gross resection with positive microscopic margin and adjuvant radiation therapy is about 75%. Typically, positive microscopic margins are treated with a boost of intraoperative, perioperative brachytherapy, or additional postoperative radiation to a total dose of about 60–70 Gy, although some recent data suggest that selected patients such as those with well-differentiated liposarcoma and a focal, planned positive margin following preoperative radiation may not benefit additional postoperative boost radiation (15). It is reasonable to assume that total gross resection of retroperitoneal tumors along with adequate doses of radiation could achieve local control rates similar to that seen for extremity tumors resected with positive microscopic margins. However, unlike the extremity, it is difficult to deliver high doses of radiation to the abdomen. The availability of intensity-modulated radiation therapy, proton beam radiation, and intraoperative radiation therapy may facilitate the efficacy and minimize morbidity of adjuvant radiation therapy for these tumors. In a report from our institution, 29 patients were treated with preoperative radiation to a median dose of 45 Gy and then underwent complete gross resection (16). Intraoperative radiation therapy (IORT) (10–20 Gy) was delivered to 16 of the 29 patients. Local control at 5 years was 83% for patients who received both preoperative and intraoperative radiation therapy and 61% for those who received only preoperative radiation. More recently, we have incorporated the use of preoperative proton beam radiation therapy and/or IMRT along with aggressive anterior surgical resection +/− and intraoperative radiation therapy for retroperitoneal tumors to further minimize to maintain high rates of local tumor control while minimizing morbidity on adjacent structures. In a report of this experience with a median follow-up of 33 months, Yoon et al noted only two local recurrences among 20 patients treated for primary retroperitoneal sarcomas. Among all 28 patients in the series, 28% had surgical complications and 14% had radiation related complications (17).
 FOLLOW-UP
FOLLOW-UP
The intensity of follow-up visits and imaging studies varies between institutions, and can also be varied according to tumor grade. The National Comprehensive Cancer Network (NCCN) published guidelines in 2011 suggesting for low-grade extremity tumors, patients should be evaluated by history and physical examination every 3–6 months for the first 2–3 years, then annually. Chest x-rays should be obtained every 6–12 months. Imaging of the primary tumor site depends on the location and the risk of locoregional recurrence. For superficial tumors, physical examination is often sufficient. For deeper extremity tumors, CT scan or MRI may be performed.
For high-grade lesions, the NCCN guidelines suggest a history a physical examination every 3–6 months for 2–3 years, then every 6 months for the next 2 years, then annually. Chest CT scans or chest x-rays should be obtained at each visit.
METASTATIC DISEASE
While local control of STS can be attained in >90% of patients, up to 50% of patients present with or develop metastatic disease. Median survival after the development of metastatic disease is 8–12 months, although a sizeable minority of patients can live several years if the disease is indolent. The most common site of metastatic disease for extremity and trunk STS is the lung. Intra-abdominal and retroperitoneal sarcomas metastasize with about equal frequency to the lung and liver. STS rarely metastasizes to regional lymph nodes (about 5%) except for certain histologic subtypes including clear cell sarcoma, epithelioid sarcoma, rhabdomyosarcoma, hemangiosarcoma, and synovial sarcoma.
As noted above, doxorubicin and ifosfamide have been demonstrated to be the most active chemotherapy agents in widely disseminated soft tissue sarcoma (18). Response rates range from about 20% to 30%, and there is some evidence that higher doses can result in increased response rates. These two agents carry significant risks of toxicity: doxorubicin dosage is limited by cardiotoxicity and ifosfamide causes hemorrhagic cystitis and nephrotoxicity. Ifosfamide-induced hemorrhagic cystitis can be avoided by adding the protective agent mesna. Another agent with some activity against STS is dacarbazine (DTIC), with reported response rates around 20%. However for metastatic disease, complete responses are uncommon (<15% even with combination therapy), duration of response averages 8 months, and as yet the higher response rates seen with intense combination therapy have not been proven to provide a survival advantage over that conferred by sequential single agent treatment.
A variety of combination chemotherapy regimens for metastatic disease have been studied in randomized clinical trials. Most of these studies were small and included mixed histologies (see Table 61-3). Most of these trials include doxorubicin (or epirubicin) and an alkylating agent. Overall survival for any combination chemotherapy regimen has not been clearly demonstrated to be superior to doxorubicin alone, but trials have been underpowered due to the rarity of this disease type. A commonly used combination chemotherapy regimen is mesna, adriamycin, ifosfamide, and dacarbazine (MAID). In a large phase II trial, this regimen achieved an overall response rate of 47%. When MAID was compared to the combination of adriamycin and dacarbazine in a randomized trial, the response rate was higher for MAID (32% vs 17%). However, there were more toxic deaths in the MAID group and there was no survival advantage to MAID. Higher doses of chemotherapy can be given with better control of toxicity if granulocyte-colony-stimulating factor (G-CSF) is used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree