Advantages
Outpatient treatment, even for tumours covering large areas
Healthy tissue can be preserved
Large safety margins possible (a decisive advantage over surgical excision margins that tend to be marginal in size, in particular in the facial region)
High effectiveness
Cosmesis usually good or very good (face > trunk and extremities)
Most functional results are excellent
Anaesthesia not required
Also possible for anticoagulation
Disadvantages
Only for selected patients
Several appointments required
No subsequent radiation treatment possible in the same location after treatment of tumours
Permanent loss of hair
Cosmetic results are inferior on the trunk and the extremities compared to the facial area
Chronic radiodermatitis tends to become more extensive over time when compared with surgical scarring
Improved X-ray technology with new devices, maintenance of safety rules and accurate dosimetry have reduced the side effects to a minimum. To achieve optimal results, knowledge of some technical issues are essential [2]. There are three main forms of radiation therapy: teletherapy (external beam radiation therapy including X-rays and electrons, brachytherapy and systemic radioisotope therapy). In dermatology teletherapy is by far most often used.
Physical Background
In addition to fast electrons used during the therapeutic application of ionising radiation in dermatology, very soft, i.e. low-energy, Grenz and soft X-rays are preferred, as these are mainly absorbed by the outermost layers of tissue. Voltages in the range of 10–50 kV are usually used to generate these X-rays, rarely up to 100 kV. The treatment of skin diseases with soft X-rays requires an understanding of some important parameters like radiation quality and homogeneity and symmetry of the radiation fields. An understanding of two basic physical laws is of great importance here: (1) inverse square law (the intensity of the dose (dose rate) of X-rays emitted from a point source is inversely proportional to the square of the distance from that source) and (2) absorption law (the absorption of x-rays is proportional to the thickness, density and atomic number of the material and inversely proportional to the energy of the x-ray quanta).
Radiation Quality
Radiation quality determines the depth of penetration of radiation into the tissue. This is set by selection of the voltage (kV) on the X-ray equipment and the associated filtering. The voltage range for soft X-ray equipment lies between 10 and 100 kV. The limit is 50 kV for most equipment used in dermatology.
Selection of additional filtering is dependent on the tube potential. Plastic foil (Cellon) is generally used in the 12 kV range of Grenz rays. Aluminium filters of different thicknesses are used for higher voltages of up to 100 kV.
In order to avoid faulty irradiation, the equipment must be fitted with a filter-safety device or with fixed voltage-filter combinations appropriate to the type of equipment. The combinations are pre-installed in the newest equipment (Fig. 27.1). In addition to information on the voltage and the selected filters, a full description of radiation quality must also include the half-value layer thickness (HVL). HVL refers to the layer thickness that reduces the intensity of the radiation to half its initial strength. It increases with hardening of the beam.
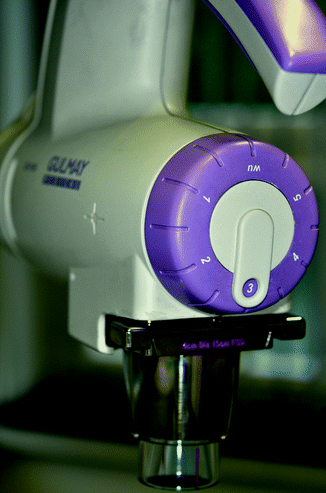

Fig. 27.1
Pre-installed voltage-filter combinations in newest equipment
Half-Value Depth in Tissue
The depth of penetration into the tissue determined by the radiation quality is usually described by the half-value depth in tissue (HVD). This is the depth in the tissue at which the intensity of the radiation or the dose rate has been reduced to half of the surface dose rate. HVD also increases with hardening of the beam. However, due to the inverse square law, it is also dependent on the focus-skin distance (FSD), i.e. on the length of the tube.
Half-value layer thicknesses can now be measured simply using foils and plates made of materials with equivalent water contents. Tables on HVL can be found in the literature. Particular care is required if cartilage or bone is located immediately under the skin in the radiation field. Low-energy X-ray quanta are mainly absorbed due to the photo effect, and this results in three to four times more energy being deposited in bone than in water or tissue located at the same depth. This can cause dangerous overdoses in the bone.
Radiation Quantity
Radiation quantity is described by the dose rate at the surface of the skin. Dosing in radiotherapy is measured using the energy dose unit Gray (1 Gy = 1 J/kg). The surface dose is dependent on several parameters. The most important parameters are tube current, voltage and filtration, as well as field size. Radiation quantity or the dose rate basically increases linearly with tube current (in mA).
A high percentage of X-ray quanta in the voltage range of up to 100 kV are backscattered due to their interactions with molecules in the tissue. This results in an increase in the surface dose for the tissue. This backscatter contribution increases with field size and voltage. It can be neglected for small fields and low voltages, whereas it can be up to 30 % at 50 kV and more for larger radiation fields.
The therapeutic approach for different skin diseases is shown in Table 27.2.
Table 27.2
Recommended doses for different skin diseases
Diagnosis | Voltage (kV) | Field (cm) | Fractionation (Gy) | Total dose (Gy) | Interval (days) |
|---|---|---|---|---|---|
BCC and SCC | 20–50 | <2 | 5–6 × 8 | 40–48 | 4–7 |
2–5 | 10–12 × 4 | 3–4 | |||
>5 | 26–28 × 2 | 52–56 | Daily | ||
Merkel cell tumour/SCCmetastasis | 20–50 | >5 | 26–28 × 2 | 52–56 | Daily |
Mycosis fungoides/otherlymphomas | 20–50 | 3–7 × 2 | 6–14 | 3–4 | |
Kaposi’s sarcoma | 20–50 | <2 | 3–5 × 8 | 24–40 | 4–7 |
>2 | 5–10 × 4 | 20–40 | 3–4 | ||
Lentigo maligna/melanomametastasis | 20–50 | 7–10 × 6 | 42–60 | 2–7 | |
Lentigo maligna | 12 | <2 | 5–6 × 20 | 100–120 | 4–7 |
>2 | 10–12 × 10 | 100–120 | 3–4 | ||
Bowen’s disease/Queyraterythroplasia | 20 | <2 | 3–4 × 8 | 24–32 | 4–7 |
>2 | 8–10 × 4 | 32–40 | 3–4 | ||
Actinic keratosis | 12 | <2 | 2–3 × 8 | 16–24 | 4–7 |
20 | >2 | 5–7 × 4 | 20–28 | 3–4 | |
Chronic eczema | 12 | 6–12 × 1 | 6–12 | 4–7 | |
20 | 6–12 × 0.5 | 3–6 | 4–7 | ||
Psoriasis | 12 | 4–12 × 2 | 8–24 | 4–7 | |
20 | 4–12 × 1 | 4–12 | 4–7 | ||
Lichen planus | 20–50 | 6–12 × 0.5 | 3–6 | 4–7 | |
Painful venous ulcer | 20–50 | 5–10 × 0.2 | 1–2 | Daily | |
Pruritus ani/vulvae | 12 | 4–8 × 1 | 4–8 | 4–7 |
Radiation Therapy for Malignant Skin Tumours
Indications
Curative Treatment
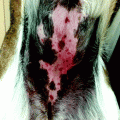
In general, patients over the age of 60 with moderately to larger-sized tumours in the facial region can be treated with radiotherapy (RT) when the expected outcome is better than with alternative methods [4]. Locations like the eyelids (Fig. 27.2), nose (Fig. 27.3), lips and ears produce particularly satisfactory results, both cosmetically and functionally. It is likewise an excellent option for patients receiving anticoagulants [4].
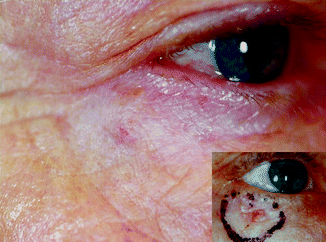
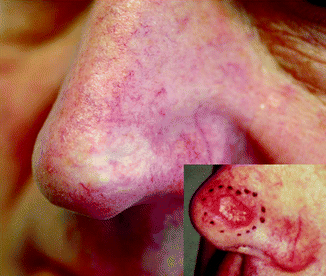

Fig. 27.2
Nodular BCC in a 67-year-old patient 4 years after 12 × 4 Gy, 30 kV

Fig. 27.3
SCC in a 64-year-old patient 5 years after 12 × 4 Gy, 40 kV
The half-value depth in tissue (HVD) must always correspond to the approximate thickness of the tumour. This usually results in optimum protection of the skin lying underneath, while simultaneously destroying the tumour. When suspecting the presence of possible invasive tumours, a magnetic resonance imaging (MRI) is required prior to therapy as tumours that have infiltrated cartilage or bone are not an indication including soft X-ray and surface therapy.
Radiation treatment is only carried out after a histopathological examination. The histology provides information not only on the type and extent of the tumour but also on potential special histological subtypes, such as sclerodermiform growth patterns in basal cell carcinomas [5]. The latter are less sensitive to radiation – probably due to the concomitant fibrosis – and often exhibit a tendency to relapses, partly due to the difficulties in clinical delimitation. However, this does not necessarily mean that radiation therapy must be excluded as an option for inoperable patients. The radiation parameters should, however, be adapted, for example, through higher individual doses or higher total doses or through treatment with faster electrons. As a rule, basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) can be treated in the same way. There is a general tendency towards using higher total doses for SCCs. Local control rates in BCCs range from 90 to 95 % [4], and careful patient selection can result in even higher cure rates [6–8]. In a prospective trial, where 93 patients with BCC were randomised to receive either cryosurgery or radiation therapy, the 2-year cure rate for the RT group was 96 % [9]. Cosmesis is rated in over 90 % as excellent or good [6]. Most important long-term side effects (after 4 years) are hypopigmentation and telangiectases in 92 and 82 %, respectively [10] (Fig. 27.4). Factors influencing cosmetic results are in order of importance: time after treatment, site, tumour thickness, field size and treatment parameters [10, 11].
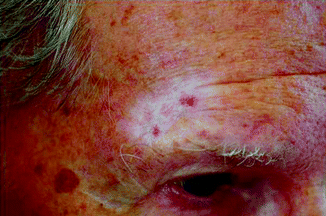

Fig. 27.4
Marked hypopigmentation and telangiectases 9 years after RT (especially in sun-exposed areas)
The risk of radiation-induced malignancy is probably lower than 1:1,000 after 10–15 years [12]. Radiotherapy can be used to treat many types of BCC, even those overlying bone and cartilage, although it is probably less suitable for the treatment of large tumours in critical sites, as very large BCC masses are often both resistant and require radiation doses that closely approach tissue tolerance.
Local control rate is lower for SCC by 10–15 % than an equivalent-sized BCC [13]. Poorly differentiated SCCs recur in up to 50 % and therefore should not be treated with X-rays [1]. For high-risk SCCs, Mohs surgery should be preferred [14].
In special circumstances – especially in larger SCCs on the lower lip – brachytherapy may be used alternatively. In these cases a radiation source is placed inside or next the tumor area. For example Cobalt-60 can be used to generate gamma rays, produced by spontaneous decay of the nucleus.
Electron beam therapy – where electrons are directed to a tumor site – is produced by a linear accelerator. An electron beam will penetrate to a certain depth and spare any underlying tissue from radiation damage. Therefore this form of radiation is best used to irradiate large skin cancers in sites overlying cartilage or bone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree