Cattle type
No. studies
Specific days of EEM
Incidence of EEM
Mean (range)
Reference
Beef heifers
2
2–16
21.8 % (4.5–43.7 %)
a
Dairy heifers
1
2–6
28.9 %
b
Beef cows
3
2–16
35.6 % (10.5–70 %)
c
Dairy cows (lactating)
1
2–7
46.3 %
d
Dairy cows (nonlactating)
3
2–7
33.3 % (13.8–46.9 %)
e
Table 13.2
Incidence of late embryonic mortality (LEM; > day 28 of gestation) in cattle
Cattle type | No. studies | No. animals | Incidence of LEM Mean (range) | Day of gestation | Reference |
|---|---|---|---|---|---|
Beef heifers | 4 | 1250 | 4.2 % (4–5 %) | 30–90 | a |
Dairy heifers | 2 | 203 | 5.4 % (4–6 %) | 30–80 | b |
Beef cows | 3 | 2570 | 10 % (6.5–14 %) | 25–65 | c |
Dairy cows (lactating) | 11 | 4680 | 14 % (3.2–42.7 %) | 27–60 | d |
13.2 Detecting the Presence of an Embryo
13.2.1 Ultrasonography
The gold standard for determining pregnancy and confirming the presence of a viable embryo is with ultrasound. In women, transvaginal ultrasound can be used to detect a gestational sac and embryonic heartbeat approximately 33 days after the last menstrual period which is detectable with a transabdominal ultrasound about a week later (Coyne and Raine-Fenning 2010). In cattle, transrectal ultrasonography can be used between days 26 and 29 to diagnose pregnancy and visualize a discernable heartbeat (Pierson and Ginther 1984; Kastelic et al. 1988; Beal et al. 1992). Today, ultrasound is considered the only visual indicator of pregnancy in cattle and is used for comparison with all recent attempts at diagnosing earlier pregnancy in this review.
13.2.2 Early Pregnancy Factor
Early pregnancy factor (EPF) or early conception factor was first reported by Morton et al. (1974) in pregnant mice, and EPF has subsequently been reported in the serum of pregnant sheep (Morton et al. 1979), pigs (Koch et al. 1983), cattle (Yoshioka et al. 1995), horses (Ohnuma et al. 2000), red deer (Lash et al. 1997), and humans (Morton et al. 1977). EPF was identified as a homologue to chaperonin 10 (Cavanagh and Morton 1994; Morton 1998) and is believed to suppress the maternal immune system, based on its activity in a rosette inhibition test (Morton et al. 1987; Morton 1998). In addition, EPF has growth factor activity and is believed to be an embryonic survival factor (see review Morton et al. 1992). In this regard, when pregnant mice were passively immunized against EPF, the pregnancy was terminated (Athanasas-Platsis et al. 1989, 1991). Prior to implantation, EPF is reportedly produced by the ovum in response to fertilization (Cavanagh et al. 1982), and postimplantation EPF is believed to be produced by the placenta (Morton 1984). Other sources of EPF include platelets in response to stimulation by platelet-activating factor, tumor cells, and regenerating liver cells (Morton 1998). Although some have suggested that EPF may be used to diagnose pregnancy in cattle, its usefulness in this regard is highly questionable (Cordoba et al. 2001). The practicality of diagnosing pregnancy prior to maternal recognition may have limited utility in a number of species due to the high incidence of early embryonic mortality in mammals. However, in cattle that are intensively managed (e.g., dairy cows), the ability to determine that a cow is not pregnant before initiation of spontaneous luteolysis would allow for resynchronization of ovulation and rebreeding at an earlier time than is currently possible.
13.2.3 INFT Stimulated Gene Expression in Ruminants
In ruminants, interferon tau (IFNT) is the primary signal for maternal recognition of pregnancy (Bazer et al. 2009; Dorniak et al. 2013). IFNT is secreted by the bovine trophoblast beginning around day 14 after insemination. It binds to a type I interferon receptor and inhibits expression of the estrogen receptor within the uterine epithelium. Inhibition of estrogen receptor transcription prevents an increase in uterine epithelial expression of oxytocin receptor, which prevents circulating oxytocin from initiating secretion of large episodic pulses of the uterine luteolysin, prostaglandin F2α (Bazer et al. 2009; Dorniak et al. 2013). IFNT was thought to act exclusively on the endometrium; however, recent evidence indicates that IFNT is released into the vasculature and has an endocrine action on ovine peripheral mononuclear blood cells (PMBC) as well as the corpus luteum (Oliveira et al. 2008; Bott et al. 2010; Hansen et al. 2010). Although IFNT has both paracrine and endocrine roles in maternal recognition of pregnancy, circulating concentrations of IFNT are too low to be detected in circulation with current assay technology. Therefore, expression of IFNT-stimulated genes in PMBC has been investigated. The upregulation of interferon-stimulated gene expression in bovine and ovine PMBCs has been investigated as the basis for the potential development of an early pregnancy test in these species (Han et al. 2006; Gifford et al. 2007; Stevenson et al. 2007; Green et al. 2010). For example, on days 16, 18, and 20 after insemination in dairy cows, expression of interferon-stimulated genes (ISG, e.g., ISG-15, Mx1, and Mx2) were increased in PBMC of pregnant compared to nonpregnant cows (ISG-15 on days 18 and 20, Mx1 on day 20, and Mx2 on days 16, 18, and 20), whereas expression of other ISGs (IFN regulatory factor 1, IFN regulatory factor 2, and β2 microglobulin in PBMC) was not different (Gifford et al. 2007). Green et al. (2010) reported differential expression of ISGs between pregnant and nonpregnant dairy cows and heifers; however, the use of ISGs to accurately diagnose pregnancy on day 18 after insemination was greater for heifers compared to cows. Since IFN-stimulated genes are not unique to pregnancy, the measurement of IFN-stimulated genes in PMBCs has proven to be more useful for identifying nonpregnant animals than it has for detecting pregnant animals.
13.2.4 Use of Steroids to Detect Pregnancy
Progesterone, a steroid hormone secreted by the corpus luteum, is essential for maintenance of pregnancy in mammals and can be detected in plasma/serum, milk, saliva, feces, and urine. Differences in serum concentrations of progesterone between pregnant and nonpregnant animals occur between the predicted time of spontaneous luteolysis and subsequent formation of a new corpus luteum in nonpregnant animals; this period represents a time in which one can measure differences in circulating concentrations of progesterone between nonpregnant and pregnant animals. The preceding divergence in concentrations of progesterone in circulation or milk formed the basis for an indirect measure of pregnancy in cattle, sheep, buffalo, horses, goats, and other species. A close association between the concentrations of progesterone in milk and plasma during the estrous cycle has been reported in dairy cows (Laing and Heap 1971; Heap et al. 1973). In cattle, there is a significant divergence in serum or milk concentrations of progesterone between nonpregnant and pregnant cows around days 20–24 post-insemination (Sasser and Ruder 1987). The accuracy associated with diagnosing pregnancy correctly on the basis of concentrations of progesterone in milk varied from 60 to 100 % (Sasser and Ruder 1987; Nebel 1988), whereas the accuracy of detecting that a cow is not pregnant based on the concentration of progesterone in milk varied from 81 to 100 % (Sasser and Ruder 1987; Nebel 1988). The reason for this discrepancy in the accuracy of the milk progesterone test is when progesterone is low between days 20 and 24 post-insemination; there is a high probability that luteolysis has occurred and the animal is not pregnant. Alternatively, the concentration of progesterone in milk may remain elevated in a nonpregnant cow between days 20 and 24 due to a longer luteal phase in cows having three versus two follicular waves, a persistent corpus luteum following uterine infection, or a luteal or luteinized cyst. Because these measures are compared to pregnancy determined at a later time, it is also likely that some early embryonic mortality existed among the cows included in the data, which may have also reduced the accuracy of diagnosing pregnancy based on progesterone in milk or circulation. The establishment of pregnancy in undomesticated species has been determined by measuring progesterone or a progestin metabolite in urine or feces of a variety of species, including big cats (Umapathy et al. 2013). However, serum concentrations of progesterone are not particularly effective at monitoring embryonic viability or the precise timing of embryonic death when it occurred after ultrasound confirmation (Pohler et al. 2013).
High correlations between blood flow in the corpus luteum (CL) and serum concentration of progesterone secretion in cattle between days 18 and 21 of gestation using Doppler ultrasonography may provide some usefulness as a chute side measure of CL viability and thus receptivity to maternal recognition of pregnancy (Ginther 2007). Detection of luteolysis using Doppler ultrasonography can be used to identify nonpregnant cattle as early as day 15, but better accuracy for the determination of pregnant cattle is achieved between days 18 and 21 (Siqueira et al. 2013; Pugliesi et al. 2014; Scully et al. 2015). While CL blood flow is an indirect measure of CL viability and serum concentrations of progesterone, it is an indirect measure that a viable embryo is present and maternal recognition of pregnancy has occurred.
Although progesterone is the primary steroid that has been used to predict pregnancy in a number of species, circulating concentrations of estrone sulfate have also been employed for this purpose (Sasser and Ruder 1987). Estrone sulfate is the form of estrogen produced by the conceptus and sulfated by the endometrium that can be detected in serum or milk (cattle) after the following days of gestation in pigs (days 20–29; (Robertson and King 1974)), sheep (day 100; (Thimonier et al. 1977)), and cattle (day 100; (Holdsworth et al. 1982)).
13.2.5 Extracellular microRNAs and Pregnancy Detection
MicroRNAs (miRNA), a class of small noncoding RNAs (approximately 22 nucleotides in length), have been shown to regulate gene expression and play critical roles in many biological systems. Generally speaking, RNAs, including miRNAs, are localized in the cytoplasm, but they can also be found in extracellular microvesicles, which may provide a mechanism for cell-to-cell communication (Valadi et al. 2007). Overall, extracellular miRNAs are encapsulated in lipid vesicles, which are released as microparticles (e.g., exosomes; (Raposo and Stoorvogel 2013)), or bound to protein, in which case the miRNA is complexed with specific proteins (e.g., high-density lipoprotein; (Vickers et al. 2011)). Exosomes are small microvesicles, ranging in size from 50 to 100 nm in diameter (Heijnen et al. 1999), and have been identified in a variety of biological fluids: urine (Pisitkun et al. 2004), amniotic fluid (Keller et al. 2007), serum (Gilad et al. 2008), human saliva, plasma, and breast milk (Lasser et al. 2011). Circulating miRNAs may serve as potential biomarkers for determinants of health status and disease (see review Reid et al. 2011). For the purpose of this review, we will focus on exosomal-derived miRNAs in the maternal circulation that are pregnancy specific.
In relation to reproduction, exosomal microRNAs have been associated with reproductive cancers, male and female reproductive tract function, and stages/health of pregnancy. Numerous microRNAs have been shown to be pregnancy specific in normal human tissue (Bentwich et al. 2005; Liang et al. 2007), and more recently, Gilad et al. (2008) demonstrated that placental-derived microRNAs can be detected in sera of pregnant, but not nonpregnant patients. All placental microRNAs mentioned above were found at increased concentrations in sera from pregnant patients and tended to increase with gestational age. Furthermore, it was demonstrated that three placental specific microRNAs (miR-526a, 527, and 520d-5p) detected in maternal circulation could accurately distinguish pregnancy status (Gilad et al. 2008) in women, thus providing a potential biomarker of pregnancy or pregnancy complications. Luo et al. (2009) demonstrated that the preceding circulating microRNAs are most likely products of human villous trophoblasts that express and secrete miRNAs into maternal circulation via exosomes. Subsequently, there have been several reports in humans that support pregnancy-specific exosomal-derived miRNAs in the maternal circulation of women (Miura et al. 2010; Kotlabova et al. 2011). An examination of the two studies identified at least five miRNAs in common that showed a significant increase in maternal circulation throughout pregnancy, followed by a rapid decrease after parturition.
Most of the investigation on the relationship between circulating microRNAs and pregnancy has been carried out in humans, which have a highly invasive placenta type, allowing for a plausible mechanism for placental specific material to enter the maternal circulation. Although it is known that fetal DNA can be found in the maternal circulation of domestic animals, it is not clear whether pregnancy-specific miRNAs are present. A recent study provided evidence that pregnancy-specific miRNAs maybe present in circulation of mares. Cameron et al. (2012) reported differences in specific exosomal-derived miRNAs between pregnant and nonpregnant mares. To date, there have been no other published reports of pregnancy-specific circulating miRNAs in domestic livestock species. However, there is evidence of miRNAs in extracellular microvesicles (potential exosomes) of uterine flushings in a ruminant species (i.e., sheep). In pregnant and cycling ewes, extracellular microvesicles, collected via uterine flushing on day 14, contained differential amounts of miRNAs and proteins (Burns et al. 2014). In addition preliminary data from our labs suggest that at days 17 and 24 of gestation, circulating microRNAs may provide a useful marker of embryonic presence (Pohler et al., unpublished data). Further research is needed in domestic animals to determine if pregnancy-specific extracellular miRNAs exist and if they can provide an accurate marker of embryonic presence and viability.
13.2.6 Detection of Placental Products to Monitor the Presence of an Embryo
The placenta is a multifaceted organ that has a vital role in the establishment and maintenance of pregnancy. Along with transferring nutrients and protecting the fetus, the placenta serves as an endocrine organ throughout pregnancy. The early embryo has been shown to produce a wide range of factors in vitro (Gardner et al. 2001); however, many of these molecules never reach a sufficient concentration in vivo for the detection in maternal circulation. In humans, detection of human chorionic gonadotropin (hCG) in circulation is the most commonly utilized marker of early pregnancy. In vitro, trophoblast cells from the developing embryo have been reported to produce hCG as early as 7 days postfertilization (Shutt and Lopata 1981; Fishel et al. 1984; Lachlan and Lopata 1988). In vivo, detection of hCG in circulation, produced by the developing embryo, has been reported to occur as early as 6.5–9.5 days after the preovulatory gonadotropin surge, which coincides with the initiation of implantation (Hay et al. 1986; Lenton and Woodward 1988). The ability to predict pregnancy loss based on circulating concentrations of hCG will be discussed in more detail in the section below.
In the ruminant placenta there is a unique cell type (giant binucleated trophoblast cells) that constitutes 15–20 % of the fetal placental epithelium (Anthony et al. 2010). Binucleated cells become visible around days 19–20 of gestation in cattle and secrete a number of hormones and proteins including placental lactogen (PL) and pregnancy-associated glycoproteins (PAGs; see Fig. 13.1; (Wooding et al. 2005)). PAGs are members of a relatively large gene family and are abundantly expressed in the placenta of species within the Cetartiodactyla order (even-toed ungulates) and can be detected in circulation and milk (Wallace et al. 2015). There is significant variation in the spatial and temporal expression of PAGs in species having an epitheliochorial and synepitheliochorial placenta. In ruminants, PAGs have gained considerable attention for the detection of pregnancy. Sasser et al. (1986) reported detectable levels of pregnancy-specific protein B (PSPB; PAG1) in the maternal circulation and developed a specific radioimmunoassay, which successfully detected pregnancy in cattle (Sasser et al. 1986), sheep (Ruder et al. 1988) and goats (Humblot et al. 1990). There has been interest in detecting other PAGs for pregnancy detection throughout gestation. Green et al. (2005) reported the establishment of an ELISA-based test for PAGs produced during early pregnancy that have a relatively short half-life (4.3 days). In the preceding study, PAGs were detected in all cattle by day 28 of gestation, PAG concentrations peaked around the time of parturition, and following parturition PAGs were undetectable by 8 weeks’ postpartum in 38 out of 40 cows. Using a similar assay platform, Pohler et al. (2013) reported an even shorter half-life for PAGs (~36 h) and determined that the first significant increase in circulating PAGs occurred on day 24 of gestation. The difference in reported half-life could be due to differences in clearance of PAGs in maternal blood during pregnancy versus the postpartum period. There are many members of the PAG family, and the presence of distinct circulating forms at different stages of gestation may account for the difference in observed half-lives between these studies. In cattle, circulating concentrations of PAGs have been shown to be influenced by a number of factors including breed, weight, parity, fetal sex, fetal number, fetal birth weight, and sire of the fetus, along with pregnancy stage and status (Patel et al. 1997; Lobago et al. 2009). Overall, PAGs have been shown to be an accurate tool for pregnancy diagnosis in cattle, sheep, goats, buffalo, and elk (Sasser et al. 1986; Sousa et al. 2006; Szafranska et al. 2006; Silva et al. 2007; Pohler et al. 2013), and several assay platforms are commercially available for both blood and milk (Leblanc 2013).
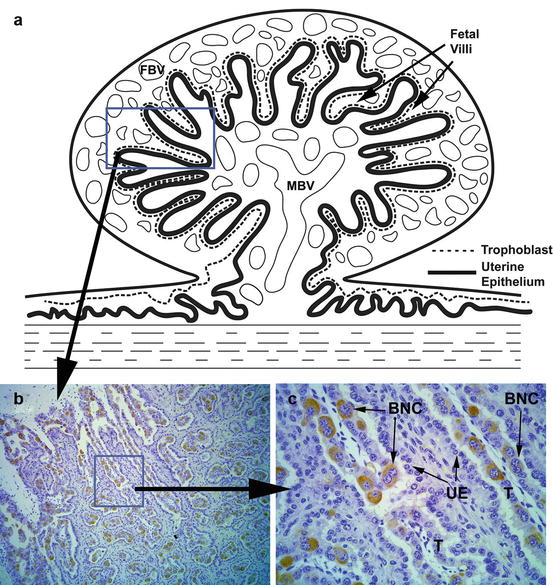

Fig. 13.1
The image illustrates the presence of trophoblast giant “binucleated” cells at the placenta-uterine interface in ruminant ungulates. (a) A stylistic drawing of a placentome, which consists of fetal villi (derived from trophoblasts and associated connective tissues) interdigitated with maternal uterine tissue. Both the fetal and maternal sides of the placentome become highly vascularized; the maternal blood vessels (MBV) and fetal blood vessels (FBV) are illustrated in the drawing. The convoluted contact area between the fetal placental trophoblasts and the uterine epithelia is represented by dashed and solid lines, respectively. (b) A section taken from a day 60 bovine placentome illustrates the interface between the trophoblasts of the placental villi and the maternal uterine epithelia. The section was incubated with an antibody raised against bovine PAG10; the resulting staining pattern reveals the presence of giant binucleated trophoblasts that comprise 15–20 % of the total trophoblast population. Magnification: 100×. (c) A higher magnification image of the boxed region shown in panel (b). The giant binucleated cells (BNC) are revealed by the immunostaining with the PAG10 antibody. The trophoblast (T) and uterine epithelium (UE) layers are indicated. Magnification: 400×
Placental lactogen (PL) has been identified and characterized in a number of species including primates, rodents, and domestic ruminants. In humans, PL was reported in circulation as early as 6 weeks of gestation and rose during the first and second trimesters, reaching a peak concentration during the third trimester (Samaan et al. 1966). Similar to PAGs, ruminant PL is produced by binucleated cells and secreted into the maternal and fetal circulation. Circulating PL is detectable in the maternal circulation of both sheep and cattle starting about day 50 of gestation (Kappes et al. 1992). Due to the low abundance of circulating PL during early to mid-gestation, little effort has been placed on using PL as a method of pregnancy diagnosis; however, later in pregnancy concentrations of PL are positively correlated with placental mass and fetal number (Gootwine 2004).
13.3 Monitoring Embryo Viability
So far, this review has focused on measurable factors, primarily in the maternal circulation, that can be used to detect the presence of an embryo. However, during the early stages of gestation, the presence of an embryo does not necessarily result in a successful pregnancy. As previously mentioned, the incidence of early embryonic mortality is relatively high during early gestation (e.g., humans = 20 %; (Macklon et al. 2002); cattle = 25 %; (Sartori et al. 2002)). Being able to predict or assess embryonic viability may provide an opportunity to intervene and to perhaps prevent embryonic mortality. Alternatively, the ability to identify an animal likely to lose a pregnancy would permit rebreeding of the animal earlier than would normally be possible. This section will focus on some methods for monitoring embryonic viability during the preimplantation and postimplantation periods.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






