Visit the Endocrine Hypertension: From Basic Science to Clinical Practice , First Edition companion web site at: https://www.elsevier.com/books-and-journals/book-companion/9780323961202 .


Introduction
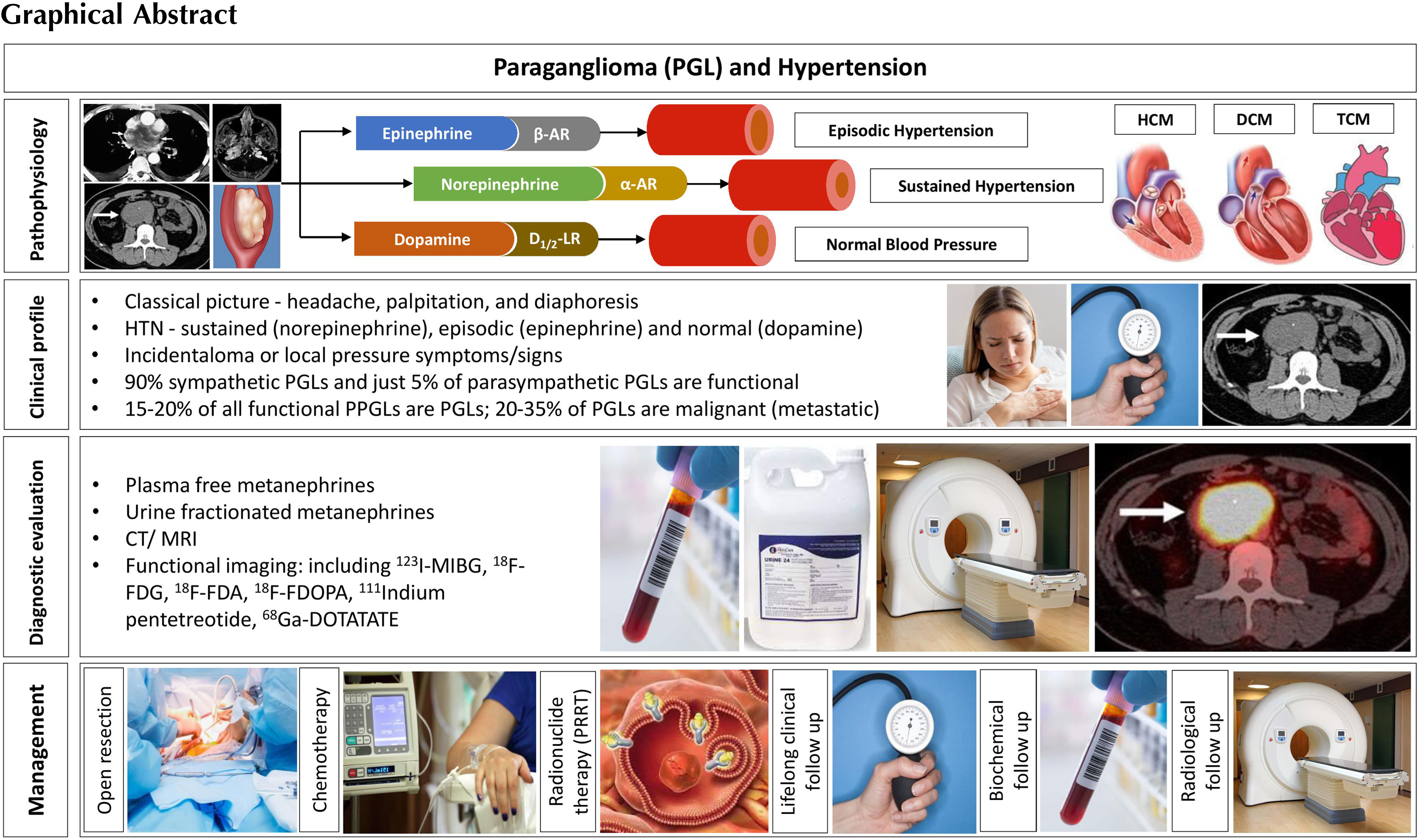
Paragangliomas (PGLs) are rare neuroendocrine tumors that arise from the extra-adrenal autonomic paraganglia, a diffuse neuroendocrine system dispersed from the skull base to the pelvic floor, derived from the embryonic neural crest cells [ ]. Paragangliomas arising from the sympathetic ganglia of the thorax, abdomen and pelvis may be catecholamine secreting, and form 15%–20% of the PPGLs (pheochromocytomas and paragangliomas), whereas paragangliomas originating from the parasympathetic ganglia of the head and neck region (HNPGLs) are often nonsecretary. While hypertension is present in the vast majority (95%) of patients with adrenal pheochromocytomas, the extraadrenal sympathetic PGLs of the thorax, abdomen and pelvis may not always present with hypertension. For example, dopamine secreting PPGLs usually present without hypertension [ ]. There are distinct differences between the adrenal pheochromocytomas and the extraadrenal PGLs in the clinical presentation, genetic characteristics, malignant potentials, management algorithms, and prognostic factors [ , ]. We outline an evidence-based review of the epidemiology, pathophysiology, genetics, clinical characteristics, diagnostic algorithms, and therapeutic aspects of PGLs in this chapter.
Epidemiology of paragangliomas and hypertension
Parasympathetic paragangliomas of the head and neck (HNPGLs) arise from the autonomic ganglia located along the glossopharyngeal and vagal nerves in the neck and at the base of the skull, and rarely within the anterior mediastinum [ ]. Generally, there are four distinct areas for the HNPGLs – carotid body, vagal, middle ear, and the larynx [ ]. The commonest among the HNPGLs are the carotid body tumors (CBTs) and are followed by jugulotympanic PGLs (JTPGLs) and vagal PGLs (VPGLs). This is followed by PGLs of larynx, trachea, thyroid, orbit, and nasal cavity [ ]. These tumor cells are chromaffin-negative (meaning that they do not stain brown when exposed to potassium dichromate), and usually
An estimated annual clinical incidence of HNPGL is 1/100,000 persons and is most frequently diagnosed in middle-aged adults (mean age 40–50 years), with equal frequency in men and women in hereditary forms, but the sporadic form is more common in women ( Table 12.1 ) [ ]. The association of chronic hypoxia in the development of carotid body PGL (the older term chemodectoma or CBT) is well known, with a higher incidence in people living at high altitudes, and in the setting of chronic obstructive pulmonary disease [ ]. Genetic cases are more than a decade younger, and often multifocal [ ]. A positive family history increases the risk of multifocality: up to 78% of the cases with a positive family history and 17%–37% of the unselected cases are multicentric [ ]. In a large series, evidence of malignancy has been found in 3%–5% of HNPGL cases, with a lower risk for carotid body and middle ear HNPGLs (2%–6%) and a higher risk for vagal paragangliomas (16%) [ ].
| Carotid body PGL | Vagal PGL | Middle ear PGL | Laryngeal PGL | |
|---|---|---|---|---|
| Localization | Carotid body | Along the nerve and nodose ganglion | Adventitia of jugular bulb or along the middle ear medial promontory wall | Superior/supraglottic (80%) and inferior paired paraganglion |
| Percentage | 60 | 10 | 30 | Very rare |
| Mean age | 50–60 | 50 | 60 | 40–60 |
| Female: Male | 2:1 (8:1 at high altitudes) | 2:1–8:1 | 3:1–9:1 | 3:1 |
| Clinical symptoms | Asymptomatic | 70% asymptomatic, <4% clinically functional | Pulsatile tinnitus, hearing loss, aural fullness | Dyspnea, hoarseness, stridor |
| Bilateral/multifocal | 10–25% | 16% | 2% | 2% |
| Hereditary | ∼33% | ∼33% | ∼33% | Unknown |
Sympathetic PGLs are chromaffin-positive tumors located in the sympathetic paravertebral ganglia of the thorax, abdomen, and pelvis that generally synthesize, store, and secrete catecholamines. They are also known as functional PGLs. Because the clinical patterns of PGLs are commonly described together with those of pheochromocytomas, the specific incidence of functional PGLs is largely unknown. The incidence of PGLs is estimated at 1/300,000 and is diagnosed in the third to fifth decades, with no gender differences. A nationwide study of PPGLs in the Netherlands revealed age-standardized incidence rates for PGLs at 0.11 (95% CI: 0.09–0.13) per 100,000 person-years [ ]. Similar to HNPGLs, hereditary paragangliomas tend to develop disease about a decade earlier than in the sporadic form [ ]. However, the incidence of these tumors is much higher at autopsy (∼0.05%), probably due to the often-asymptomatic clinical course [ ]. Of these neuroendocrine tumors (PPGLs), 80%–85% are pheochromocytomas, and 15%–20% are PGLs [ ]. The vast majority of functional PGLs (70%–80%) arises from the infradiaphragmatic sympathetic ganglia in the abdomen, most often at the junction of the vena cava and the left renal vein, or at the organ of Zuckerkandl located near the origin of the inferior mesenteric artery at the aortic bifurcation. It is less frequently found in the retroperitoneal area around the kidney and adrenal, and in the bladder. Approximately 10% of PGLs are in the mediastinal and pericardial locations within the thorax. Sympathetic PGLs can very rarely also arise in the thyroid gland, adjacent to the thoracic spine, and at the level of the cauda equina [ ].
About 26% of PGLs are multiple, and one-third to one-half are associated with a hereditary syndrome. Multiplicity is far more common in hereditary cases [ ]. The incidence of malignancy depends on the genetic background and anatomical location [ ]. Approximately 20% of the secretory PGLs (abdominal and mediastinal) are malignant. The highest malignancy rates are seen in PGLs associated with SDHB mutation, which are usually abdominal and secretory [ ].
Pathophysiology of paraganglioma and hypertension
There exists distinct secretory pathways in PPGLs. Eisenhofer et al . showed that the norepinephrine-producing tumor is associated with less maturity of chromaffin cells with a constitutive form of secretion, and a reduced response to secretagogue stimuli, whereas the epinephrine-producing tumor is related to a regulated secretory pathway and higher sensitivity to various stimuli [ ]. Chromaffin cells of the secreting PGLs do not have the ability to produce epinephrine, compared to the adrenal medulla, due to a lack of the relevant enzyme machinery.
Norepinephrine acts primarily on alpha-adrenoceptors and induces vasoconstriction of vessels, volume contraction with an increased peripheral vascular resistance, and leads to sustained arterial hypertension [ ]. Dopamine targets D 1 and D 2 dopaminergic receptors. Activation of D 1 receptors results in vasodilation of the renal arteries, while D2 activation will inhibit norepinephrine secretion from sympathetic nerve terminals and have a mild negative inotropic effect on the heart [ ]. The signaling net result would explain the clinical phenomenon of lack of hypertension and palpitations in patients with dopamine secreting PGLs.
While normal levels of dopamine do not affect the adrenergic receptors, it can stimulate both α and β adrenoceptors when dopamine increases in the circulation, as in the case of tumors that exclusively secrete dopamine. Dopamine overproduction may be due to further tumor dedifferentiation and may indicate a malignant potential for PGLs [ ].
Desensitization of adrenergic receptors has always been a hot topic in both research and clinical care. There have been numerous studies that reported significant desensitization of both α and β adrenergic receptors in either healthy humans or patients with PPGLs as well as in animal models [ ].
Genetics of paragangliomas
PGLs are tumors which present with a high degree of heritability, perhaps the highest among other cancers. More than 40% of cases may carry a germline mutation—its prevalence depends mostly on the population studied: for example, it is very high in the Netherlands, due to the presence of a founder mutation of the SDHD gene. During the last 2 decades, more than 20 susceptibility genes associated with the development of PGLs or pheochromocytoma have been identified. According to the Cancer Genome Atlas, these genes were divided into three distinct molecular groups: Pseudohypoxia-related (Cluster 1), and Kinase signaling-related (Cluster 2), and Wnt-related (Cluster 3) [ , ]. Unlike pheochromocytoma, which is associated with gene mutations of all three groups, the vast majority of PGLs is caused by mutations of the Cluster 1-related genes. Only a few PGL cases have been published in the kinase signaling group and none in the Cluster 3 [ ].
The pseudohypoxic group is characterized by activation of pathways which mimic hypoxia signaling. It is divided into two subgroups—according to the position of the gene mutation, either in the Krebs cycle (Cluster 1A) or the hypoxia-signaling pathway (Cluster 1B). Cluster 1A-related genes include succinate dehydrogenase subunits ( SDHx ), succinate dehydrogenase complex assembly factor 2 ( SDHAF2 ), fumarate hydrogenase ( FH ), malate dehydrogenase 2 ( MDH2 ), mitochondrial glutamic-oxaloacetic transaminase ( GOT2 ), 2-oxoglutarate-malate carrier ( SLC25A11 ), dihydrolipoamide S-succinyl transferase ( DLST ), and isocitrate dehydrogenase1 ( IDH1 ). The Cluster 1B-related group consists of mutations of Egl-9 prolyl hydroxylase-1 and -2 ( EGLN1/2 encoding PHD1/2), von Hippel–Lindau ( VHL ) tumor suppressor, hypoxia-inducible factor 2α ( HIF2A/EPAS1 ) and iron regulatory protein 1 ( IRP1 ) genes [ ]. Moreover, mutations in other genes associated with PGLs (alone or in combination with pheochromocytoma), such as genes involved in DNA hypermethylation: histone subunit gene ( H3F3A ) [ ], and DNA methyltransferase ( DNMT3A ), have been described [ ].
In Cluster 2, PGLs were described in a few subjects with multiple endocrine neoplasia type 2 [mutation in the rearranged-during-transfection ( RET ) proto-oncogene], or with mutation in the MERTK gene [ ]. Interestingly, somatic mutations of the HRAS and FGFR1 genes were described predominantly in Chinese patients with epinephrine producing PGLs, but not in the European population [ ].
In contrast to pheochromocytoma (Cluster 2-related genes), in which mutations of involved genes may occur as germline or somatic, most PGLs-related mutations are reported only as germline (except for Cluster 1B-related, which are more frequently somatic). Underlining mutations predispose its carrier to specific tumor development (HNPGLs or sympathetic PGLs), metastatic or multifocal involvement, disease penetration, and other syndrome-related tumors ( Table 12.2 ).
| Gene | Type | Germline/Somatic | Inheritance | Syndrome | Presentation | Lifetime penetrance | Biochemical phenotype | PET | Other presentation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HNPGL | TPGL | APPGL | PHEO | Metastases | |||||||||
| Pseudohypoxic—Tricarboxylic acid cycle—related | |||||||||||||
| SDHA (succinate dehydrogenase complex flavoprotein subunit A) | TS | Germline | AD | FPGL5 | + | + | + | + | ++ | 1.7% | NA/D | 68 Ga-DOTATATE PET/CT | GIST, pituitary adenomas, pulmonary chordomas, RCC a |
| SDHB (succinate dehydrogenase complex iron sulfur subunit B | TS | Germline | AD | FPGL4 | ++ | ++ | +++ | + | +++ | 22% | NA/D | 68 Ga-DOTATATE PET/CT | |
| SDHC (succinate dehydrogenase complex subunit C) | TS | Germline | AD | FPGL3 | ++ | +/− | + | +/− | + | 8.3% | NA/D | 68 Ga-DOTATATE PET/CT | |
| SDHD (succinate dehydrogenase complex subunit D) | TS | Germline | AD, paternal | FPGL1 | +++ (multifocal) | + | + | + | + | 43,2% b | NA/D | 68 Ga-DOTATATE PET/CT | |
| SDHAF2 (succinate dehydrogenase complex assembly factor 2) | TS | Germline | AD, paternal | FPGL2 | +++ (multifocal) | +/− | +/− | +/− | – | NA/D | 68 Ga-DOTATATE PET/CT | ||
| FH (fumarate hydratase) | TS | Germline | AD | +/− | + | + | + | + | NA/D | 18F-DOPA PET/CT | Leiomyomatosis, RCC | ||
| Pseudohypoxic group—VHL/EPAS | |||||||||||||
| VHL (von Hippel–Lindau tumor suppressor) | TS | Germline/Somatic | AD | Von Hippel-Lindau | +/− | +/− | +/− | +++ (bilateral) | +/− | 15%–20% | NA | 18F-DOPA PET/CT | Hemangioblastoma, RCC, epididymal cystadenoma, pancreatic Neuroendocrine tumors, retinal Abnormalities |
| EPAS 1 (Endothelial PAS domain protein 1) | OG | Postzygotic/somatic | Pacák-Zhuang | +/− | + | +++ | +++ | + | NA | 18F-DOPA PET/CT | Polycythemia, somatostatinoma, retinal abnormalities, organ cysts | ||
a Patients with FPGL due to mutations of SDHA-SDHD genes may present with Carney Stratakis syndrome (PGL and GIST) or Carney syndrome (PGL, GIST and pulmonary chondroma).
All subjects with PGLs should be involved in the discussion about genetic testing, irrespective of age or tumor location. This is performed using next-generation sequencing, either on DNA acquired from peripheral blood or buccal swab or, more recently, from tumor tissue. The latter approach enables not only the germline mutations (mutation found in tumorous and peripheral DNA) to be identified, but also somatic mutations (mutation found only in tumorous DNA) [ , ]. Another way of screening for SDHx mutations is SDHA/SDHB immunohistochemistry [ ]. Genetic testing allows earlier detection of primary tumors or metastases, and has a positive impact on the prognosis of subjects with SDHx or VHL mutation [ ]. Screening for tumors should be started between 6 and 10 years of age in asymptomatic SDHB mutation carriers (first PGLs reported at the age of 6), and between 10 and 15 years in SDHA, SDHC and SDHD mutation carriers [ ].
Compared to SDHD mutation carriers, which have a normal standardized mortality ratio (SMR) even in affected subjects, SDHB mutation carriers have an increased mortality rate (SMR of 1.89 increasing to 2.88 in subjects with PGLs), due to susceptibility for metastatic disease [ ].
Clinical presentation
Paragangliomas are clinically heterogenous in their presentation [ ]. Initial symptoms vary widely and can be caused by local growth. HNPGLs typically present as slow-growing, painless lateral neck masses [ , ]. In advanced disease, there can be hoarseness, dysphonia, dysphagia, hearing loss, shoulder weakness/pain, and dysarthria. Horner’s syndrome and facial paralysis may be present due to the involvement of the cranial (VII and IX–XII) or cervical nerves. Pulsatile tinnitus may also be appreciated on the side of the tumor, caused by the high-flow state due to hypervascularization. Physical examination often demonstrates a pulsatile lateral neck mass that is characteristically less mobile in the cephalocaudal direction, due to adherence to the carotid artery ( Table 12.1 ) [ ].
Another clinical attribute comes from catecholamine synthesis and release, which are inherently linked to cell differentiation. Functional PGLs secrete the catecholamines, norepinephrine and/or dopamine. In the case of norepinephrine overproduction, arterial hypertension is typical, either sustained or paroxysmal with characteristic, but nonspecific symptoms such as episodic headache, sweating, and palpitations [ ]. Patients with pure norepinephrine phenotype usually present with sustained and more severe hypertension as compared to adrenergic subtypes [ ]. Hypertension is very often characterized by an enhanced blood pressure (BP) variability, and impaired circadian BP rhythm [ , ]. Most patients with concomitant dopamine overproduction may be normotensive due to the vasodilatory actions of dopamine that might counteract the vasoconstrictor actions of norepinephrine. In patients with dopamine-secreting PGLs, tumors are usually discovered either as an incidental finding on imaging studies for an unrelated condition, or because of space-occupying complications of the lesions [ ]. Primary tumors are generally larger, and often metastatic. Presumably, the attainment of a large size in such tumors, compared with those more usually encountered, reflects the relatively mild signs and symptoms associated with predominantly dopamine-secreting tumors [ ]. Nausea, sometimes accompanied by vomiting, may be related to the emetic effects of dopamine [ ]. Table 12.3 shows the variety of clinical symptoms and signs of PGLs [ ].
| Organ system | Clinical signs and symptoms |
|---|---|
| Cardiovascular | Paroxysmal or sustained arterial hypertension Hypotension including postural hypotension Tachycardia Shortness of breath Chest pain Episodic syncope |
| Gastrointestinal | Obstipation Dry mouth Dysphagia Nausea and vomiting Abdominal pain |
| Neurologic | Tremors or shakiness Cephalgia Tingling of fingers |
| Psychiatric | Panic symptoms Fidgetiness |
| Musculoskeletal | Generalized fatigue |
| Urologic | Micturition symptoms |
| Skin | Diaphoresis (sweating) Extreme paleness in the face Clammy skin |
| Endocrine and metabolic | Hyperglycemia Weight loss Polyuria and polydipsia Fever |
Episodes of symptoms caused by PGLs can occur at any time. They can also be triggered by various stimuli as shown in Table 12.4 .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree