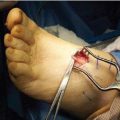
Figure 21.30 Clinical photos and radiographs of Case 4, revealing nonunion and the forefoot abduction and medial column failure (A–D). Intraoperative photos of external fixation with a forefoot and heel ring (E,F). (continued)
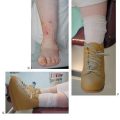
Figure 21.30 (Continued) This allows for multiple forefoot wires and adjustable compression during the postoperative period. Immediate postoperative (G,H) and three months after fixator removal (I,J) with improved forefoot abduction and medial column fault.
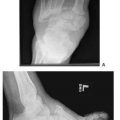
Clinically, she had failure of the medial column and forefoot abduction with callous under the first metatarsal cuneiform joint. Previous incisions medial and dorsal midfoot were well healed. Radiographs revealed an attempted Lisfranc fusion with internal fixation. The talar-first metatarsal angle on anterior-posterior radiographs measured 42 degrees of forefoot abduction, and a fault was noted on the lateral radiographs at the first metatarsal cuneiform joint. Revision was planned with removal of the hardware, cultures, reduction of the sagittal and transverse plane deformities, and application of external fixation.
The original incisions were used and the hardware removed. Cultures and biopsies were taken. A bicorrectional osteotomy was then performed with axis guides and fluoroscopy. Temporary fixation was achieved with Steinman pins and the wounds closed. The external fixator was applied using standard technique. The final configuration consisted of a tibial segment, and a foot segment with a separate heel and forefoot ring. This configuration would allow for continued compression during the postoperative course. Intraoperative cultures were positive, and she was treated with the appropriate antibiotics and further consultation with the infectious disease team. The external fixator was removed at 3 months and she progressed with our standard postoperative protocol without any complications.
CASE 5
An elderly woman from a nursing home was referred to our center with a history of an ankle fracture that occurred 2 weeks prior to her visit and during transfer to her bed (Fig. 21.31). It was treated conservatively in a posterior splint; on follow-up, the deformity had progressed, along with onset of pressure sores on her heel, ankle, and plantar aspect of the foot from the posterior splint. She had a history of diabetes mellitus and neuropathy but was ambulatory.
Figure 21.31 Initial clinical photos of Case 5, showing the unstable deformity and decubitus ulcerations (A,B). The initial radiographs (C,D) and repeat films at 2 weeks (E,F). (continued)
Figure 21.31 (Continued) Note the significant progression of the trimalleolar fracture and posterior ankle dislocation. Intraoperative fixation showing initial alignment with towels posterior (G), and axial tibial wire placement (H). The posterior malleolus was reduced with an olive wire placed posterior to anterior (I) and then attached to the foot plate (J,K). (continued)
Figure 21.31 (Continued) Final radiographs with fracture reduction (L,M).
On physical examination she had gross instability of her ankle and three preulcerative areas with eschar on the posterior heel, anterior ankle, and plantar fourth metatarsal head. There were no drainage or signs of infection. The most recent radiographs revealed a posterior dislocated trimalleolar ankle fracture. This was a limb-threatening unstable fracture in a diabetic patient, with wounds that would not heal with this deformity.
A closed reduction with external fixation was performed. The ankle was grossly reduced and a transfixation pin placed. This was done initially to hold reduction and later for added stability. A standard external fixator was applied to the leg and foot. It consisted of two tibial rings and a foot plate. Once applied, the ankle was distracted with the connecting rods to aid fracture reduction. Next, the posterior malleolus was reduced with the aid of an olive wire placed posterior to anterior. It was pulled anterior until reduction noted and attached to the foot plate. The external fixator allowed stability for the fractures and wounds. It also allowed access for a negative pressure wound therapy (NPWT) that was used on the heel and ankle postoperatively.
TIPS/PEARLS
Be efficient with intraoperative fluoroscopy to achieve the images you need, but limit exposure.
Plan osteotomies and fixation methods preoperatively.
Make sure osteotomies are complete for later gradual correction.
Take intraoperative cultures and send biopsies and frozen sections.
Increase fixation stability in diabetic ankle fractures.
Increase non–weight-bearing period and clinic visits in diabetic ankle fractures.
Be prepared for change in fixation plans, and have equipment available.
Check that skinny wires bend at the correct cortical margin to ensure placement.
Use olive wires to increae stability and aid in reduction.
Add more connecting rods, wires, and half pins at the end of each case.
Check all connection parts and fixation at the end of the case.
Use a half pin as a “joystick” to aid in calcaneal reduction.
Do not torque the intramedullary nail jig while placing screws.
Check in 2 views to ensure intramedullary screw placement.
Use long guide wires for reaming the tibial canal.
CONCLUSION
The surgical management of malunions and nonunions of the diabetic neuropathic lower extremity poses a great challenge to the reconstructive surgeon. These complex cases require a team of medical and surgical specialties with interest in diabetic limb salvage. Patient and family education, prevention combined with a vast knowledge, and surgical experience with external fixation and preoperative planning will provide successful long-term outcomes.
REFERENCES
- Alvarez RG, Barbour TM, Perkins TD. Tibiocalcaneal arthrodesis for non-braceable neuropathic ankle deformity. Foot Ankle Int 1994;15(7):354–359.
- Brink DS, Eickmeier KM, Levitsky DR, et al. Subtalar and talonavicular joint dislocation as presentation of diabetic neuropathic arthropathy with salvage by triple arthrodesis. J Foot Ankle Surg 1994;33(6):583–589.
- Brodsky JW. The diabetic foot. In: Mann RA, Coughlin MJ, eds. Surgery of the foot and ankle. St. Louis: Mosby-Year Book, 1993:877–958.
- Caravaggi C, Cimmino M, Caruso S, et al. Intramedullary compressive nail fixation for the treatment of severe Charcot deformity of the ankle and rearfoot. J Foot Ankle Surg 2006;45(1):20–24.
- Chantelau E. The perils of procrastination: effect of early vs. delayed detection and treatment of incipient Charcot fracture. Diabetic Med 2005;22:1707–1712.
- Drennan DB, Fahey JJ, Maylahn DJ. Important factors in achieving arthrodesis of the Charcot knee. J Bone Joint Surg Am 1971;53-A(6):1180–1193.
- Eichenholz SN. Charcot joints. Springfield, IL: Charles C Thomas, 1966.
- Fabrin J, Larsen K, Holstein PE. Arthrodesis with external fixation in the unstable or misaligned Charcot ankle in patients with diabetes mellitus. Int J Low Extrem Wounds 2007;6(2):102–107.
- Jani MM, Ricci WM, Borrelli J Jr, et al. A protocol for treatment of unstable ankle fractures using transarticular fixation in patients with diabetes mellitus and loss of protective sensibility. Foot Ankle Int 2003;24(11):838–844.
- Moore TJ, Prince R, Pochatko D, et al. Retrograde intramedullary nailing for ankle arthrodesis. Foot Ankle Int 1995;16(7):433–436.
- Mueller MJ, Sinacore DR, Hastings MK, et al. Impact of Achilles tendon lengthening on functional limitations and perceived disability in people with a neuropathic plantar ulcer. Diabetes Care 2004;27(7):1559–1564.
- Myerson MS, Edwards WH. Management of neuropathic fractures in the foot and ankle. J Am Acad Orthop Surg 1999;(7):8–18.
- Myerson MS. Diabetic neuroarthropathy. In: Myerson MS, ed. Foot and ankle disorders. Philadelphia: Saunders, 2000:439–465.
- Myerson MS, Alvarez RG, Lam PW. Tibiocalcaneal arthrodesis for the management of severe ankle and hindfoot deformities. Foot Ankle Int 2000;21(8):643–650.
- Paley D. Principles of deformity correction. New York: Springer, 2002.
- Papa J, Myerson MS, Girard P. Salvage, with arthrodesis, in intractable diabetic neuropathic arthropathy of the foot and ankle. J Bone Joint Surg Am 1993;75-A(7):1056–1066.
- Pelton K, Hofer JK, Thordarson DB. Tibiocalcaneal arthrodesis using a dynamically locked retrograde intramedullary nail. Foot Ankle Int 2006;27(10):759–763.
- Pinzur MS. Surgical versus accommodative treatment for Charcot arthropathy of the midfoot. Foot Ankle Int 2004;25(8):545–549.
- Pinzur MS, Sage R, Stuck R, et al. A treatment algorithm for neuropathic (Charcot) midfoot deformity. Foot Ankle Int 1993;14(4):189–197.
- Pinzur MS, Lio T, Posner M. Treatment of Eichenholz stage 1 Charcot foot arthropathy with a weightbearing total contact cast. Foot Ankle Int 2006;27(5):324–329.
- Pinzur MS. Benchmark analysis of diabetic patients with neuropathic (Charcot) foot deformity. Foot Ankle Int 1999;20(9):564–567.
- Prisk VR, Wukich DK. Ankle fractures in diabetics. Foot Ankle Clin 2006;11(4):849–863.
- Roukis TS, Zgonis T. The management of acute Charcot fracture-dislocations with the Taylor’s spatial external fixation system. Clin Podiatr Med Surg 2006;23(2):467–483.
- Saltzman CL, Hagy ML, Zimmerman B, et al. How effective is intensive nonoperative initial treatment of patients with diabetes and Charcot arthropathy of the feet? Clin Orthop Relat Res 2005;(435):185–190.
- Sanders LJ, Frykberg RG. The high risk foot in diabetes mellitus. New York: Churchill-Livingstone, 1991.
- Schon LC, Marks RM. The management of neuroarthropathic fracture-dislocations in the diabetic patient. Orthop Clin North Am 1995;26:375–392.
- Shibata T, Tada K, Hashizume C. The result of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg Am 1990;72(5):749–756.
- Stuart MJ, Morrey BF. Arthrodesis of the diabetic neuropathic ankle joint. Clin Orthop Relat Res 1990;253:209–211.
- Zarutsky E, Rush SM, Schuberth JM. The use of circular wire external fixation in the treatment of salvage ankle arthrodesis. J Foot Ankle Surg 2005;44(1):22–31.
INTRODUCTION
Bone grafting as a concept started out as simple experiments in an attempt to save lives. It has progressed over the last 400 years as an essential tool for the reconstructive surgeon. To fully benefit your patients and achieve optimal results with profoundly complex surgeries, each surgeon must keep up to date on current grafting concepts and also have a full and working knowledge of the varieties, availability, and applications of orthobiologics.
This chapter comprises both clinical and surgical applications of bone grafting concepts and orthobiologics. The emphasis is on useful surgical applications.
TERMS AND VOCABULARY
 Autograft: Cortical or cancellous bone graft harvest from the same patient.
Autograft: Cortical or cancellous bone graft harvest from the same patient.
Examples: Iliac crest, proximal tibia, distal tibia, calcaneus
 Allograft: Bone harvested from another human. It is processed and supplied in various forms. Examples: Cancellous bone chips, demineralized bone matrix, freeze dried bone grafts, fresh frozen graft
Allograft: Bone harvested from another human. It is processed and supplied in various forms. Examples: Cancellous bone chips, demineralized bone matrix, freeze dried bone grafts, fresh frozen graft
 Bone morphogenic protein (BMP): Protein substances found within bone matrix that stimulate bone production through chemical signals and enzymes to affect chondrocytes, mesenchymal stem cells, endothelial cells, and osteoblasts. Various orthobiologic products containing BMPs would be osteoinductive. Examples: DBM (variable in amounts of BMP), BMP-2 (Infuse, Medtronic Sofamor Danek, Memphis, TN), and BMP-7 osteogenic protein 1 (OP-1, Stryker Biotech, Hopkinton, MA)
Bone morphogenic protein (BMP): Protein substances found within bone matrix that stimulate bone production through chemical signals and enzymes to affect chondrocytes, mesenchymal stem cells, endothelial cells, and osteoblasts. Various orthobiologic products containing BMPs would be osteoinductive. Examples: DBM (variable in amounts of BMP), BMP-2 (Infuse, Medtronic Sofamor Danek, Memphis, TN), and BMP-7 osteogenic protein 1 (OP-1, Stryker Biotech, Hopkinton, MA)
 Calcium ceramics: Purely osteoconductive component of hydroxyapatite and mineral crystals that can be manufactured and processed to mimic the lattice of human bone.
Calcium ceramics: Purely osteoconductive component of hydroxyapatite and mineral crystals that can be manufactured and processed to mimic the lattice of human bone.
 Calcium phosphate: A group of graft substitutes that are purely osteoconductive. They are mixtures of different calcium phosphates which can also be combined with other components of hydroxyapatite. The biological and mechanical differences are based on the differing chemical structures.
Calcium phosphate: A group of graft substitutes that are purely osteoconductive. They are mixtures of different calcium phosphates which can also be combined with other components of hydroxyapatite. The biological and mechanical differences are based on the differing chemical structures.
Examples: Alpha-BSM (DePuy, Warsaw, IN), Norian SRS (Norian Corp., Cupertino, CA), Vitoss (Orthovita, Malvern, PA), Conduit (DePuy, Warsaw, IN)
 Calcium sulfate: Calcium-based group of materials that degrade fairly rapidly. They provide minimal osteoconductive scaffold because of their rapid dissolution. They are good transporters of heat-stable antibiotics. Examples: Osteoset (Wright Medical Products, Nashville, TN), BonePlast (Interpore Cross, Irvine, CA), and JAX (Smith & Nephew, Memphis, TN)
Calcium sulfate: Calcium-based group of materials that degrade fairly rapidly. They provide minimal osteoconductive scaffold because of their rapid dissolution. They are good transporters of heat-stable antibiotics. Examples: Osteoset (Wright Medical Products, Nashville, TN), BonePlast (Interpore Cross, Irvine, CA), and JAX (Smith & Nephew, Memphis, TN)
 Collagen materials: Combining collagen with calcium phosphates and HA. Examples: Healos (DePuy, Warsaw, IN) and Collagraft (Zimmer, Warsaw, IN)
Collagen materials: Combining collagen with calcium phosphates and HA. Examples: Healos (DePuy, Warsaw, IN) and Collagraft (Zimmer, Warsaw, IN)
 Demineralized bone matrix (DBM): The composite material processes from cortical and cancellous allograft bone. Different DBM products vary in the carrier of the product and especially in the amount and type of BMPs. They are freeze-dried and are osteoinductive based on their BMP levels. Examples: Accell DBM100 and Accell Connexus (IsoTis, Irvine, CA), AlloCraftTM (Stryker Howmedica Osteonics, Allendale, NJ), AlloMatrix (Wright Medical, Arlington, TN), DBX (Synthes, Paoli, PA), Grafton (Osteotech, Eatontown, NJ), InterGro TM (Interpore Cross, Irvine, CA), DynaGraft & Osteofil (GenSci Regeneration Sciences and Innova Technologies Corp., Toronto, Ontario, Canada).
Demineralized bone matrix (DBM): The composite material processes from cortical and cancellous allograft bone. Different DBM products vary in the carrier of the product and especially in the amount and type of BMPs. They are freeze-dried and are osteoinductive based on their BMP levels. Examples: Accell DBM100 and Accell Connexus (IsoTis, Irvine, CA), AlloCraftTM (Stryker Howmedica Osteonics, Allendale, NJ), AlloMatrix (Wright Medical, Arlington, TN), DBX (Synthes, Paoli, PA), Grafton (Osteotech, Eatontown, NJ), InterGro TM (Interpore Cross, Irvine, CA), DynaGraft & Osteofil (GenSci Regeneration Sciences and Innova Technologies Corp., Toronto, Ontario, Canada).
 Hydroxyapatite (HA): Grafts and materials that are processed from the exoskeletons of Gonioptera species of coral. These products come in various pore sizes to mimic cortical and cancellous bone structure. HA is purely osteoconductive and has excellent biocompatibility. HA is considered a xenograft. Examples: ProOsteon 200R and 500R (Interpore Cross, Irvine, CA). HA coating has been used in external fixation pins to help provide stability at the pin to bone interface.
Hydroxyapatite (HA): Grafts and materials that are processed from the exoskeletons of Gonioptera species of coral. These products come in various pore sizes to mimic cortical and cancellous bone structure. HA is purely osteoconductive and has excellent biocompatibility. HA is considered a xenograft. Examples: ProOsteon 200R and 500R (Interpore Cross, Irvine, CA). HA coating has been used in external fixation pins to help provide stability at the pin to bone interface.
 Mesenchymal stem cells (MSCs): These are processed stem cells that have the ability to stimulate all aspects of bone healing on a cellular level. They are osteoprogenitor or precursor parent cells to osteoblasts, osteocytes, hematopoietic stem cells, and osteoclasts. MSCs can be delivered by attaching them to cancellous allograft. MSCs have properties of osteogenesis and osteoinduction. Example: OsteoCel (Osiris Therapeutics, Baltimore, MD)
Mesenchymal stem cells (MSCs): These are processed stem cells that have the ability to stimulate all aspects of bone healing on a cellular level. They are osteoprogenitor or precursor parent cells to osteoblasts, osteocytes, hematopoietic stem cells, and osteoclasts. MSCs can be delivered by attaching them to cancellous allograft. MSCs have properties of osteogenesis and osteoinduction. Example: OsteoCel (Osiris Therapeutics, Baltimore, MD)
 Orthobiologics/osteobiologics: The group of synthetic materials that mimic bone autograft and its desirable characteristics. They may also be combination of allograft and synthetic materials. Examples: Calcium ceramics, calcium phosphate: Vitoss (Orthovita, Malvern, PA), Conduit (DePuy, Warsaw, IN), calcium sulfate: OsteoSet (Wright Medical, Arlington, TN), BonePlast (Interpore Cross, Warsaw, IN), and JAX (Smith & Nephew, Memphis, TN), Calcium cements: Norian SRS (Norian Corp., Cupertino, CA) and alpha-BSM (DePuy, Warsaw, IN), HA: ProOsteon 200R and 500R (Interpore Cross, Irvine, CA). DBM and BMPs may be included as a broad definition
Orthobiologics/osteobiologics: The group of synthetic materials that mimic bone autograft and its desirable characteristics. They may also be combination of allograft and synthetic materials. Examples: Calcium ceramics, calcium phosphate: Vitoss (Orthovita, Malvern, PA), Conduit (DePuy, Warsaw, IN), calcium sulfate: OsteoSet (Wright Medical, Arlington, TN), BonePlast (Interpore Cross, Warsaw, IN), and JAX (Smith & Nephew, Memphis, TN), Calcium cements: Norian SRS (Norian Corp., Cupertino, CA) and alpha-BSM (DePuy, Warsaw, IN), HA: ProOsteon 200R and 500R (Interpore Cross, Irvine, CA). DBM and BMPs may be included as a broad definition
 Osteoconductive: To serve a bridge or framework for the laying down and the building of new bone by the ability to stimulate the attachment and migration of osteogenic cells. Examples: Cancellous allograft chips, HA, and tricalcium phosphates
Osteoconductive: To serve a bridge or framework for the laying down and the building of new bone by the ability to stimulate the attachment and migration of osteogenic cells. Examples: Cancellous allograft chips, HA, and tricalcium phosphates
 Osteoinductive: Ability of a material or substance to signal and stimulate host cells to produce bone. Examples: DBM and BMP Osteogenic: Ability to induce osteoid and mineralized matrix through differentiated osteoblasts and osteocytes independently. Examples: MSCs and autograft bone marrow aspirate Platelet gel concentrate: Concentrated growth factors derived from autograft platelets. This concentrate of spun down platelet granules assist in new tissue formation by mediating chemotaxis, cell adhesion, and new formation or osteoprogenitor cells. Examples: Symphony Platelet Concentrate System (DePuy, Warsaw, IN), Interpore Cross AGF system (Interpore Cross, Warsaw, IN)
Osteoinductive: Ability of a material or substance to signal and stimulate host cells to produce bone. Examples: DBM and BMP Osteogenic: Ability to induce osteoid and mineralized matrix through differentiated osteoblasts and osteocytes independently. Examples: MSCs and autograft bone marrow aspirate Platelet gel concentrate: Concentrated growth factors derived from autograft platelets. This concentrate of spun down platelet granules assist in new tissue formation by mediating chemotaxis, cell adhesion, and new formation or osteoprogenitor cells. Examples: Symphony Platelet Concentrate System (DePuy, Warsaw, IN), Interpore Cross AGF system (Interpore Cross, Warsaw, IN)
 Xenograft: Different species graft such as coral or HA (1–24).
Xenograft: Different species graft such as coral or HA (1–24).
AUTOGRAFTS
Autografts, or a same-person donor, have been considered the gold standard for bone grafting and have stood this test over time. Their lack of immunologic reactions, availability, and ideal molecular characteristics has been unmatched. This has only been rivaled by the morbidity of graft site harvest, the limited amounts available for procurement and the ease of obtaining both bone bank allograft and orthobiologic materials. Advancement in stem cell research and other orthobiologics will continue to challenge the standards in surgical grafting (1,3,5,12,25–29).
Orthobiologically enhanced allograft materials have allowed many of the same benefits as that of autografts. Many surgeons also combine autografts with orthobiologic/allograft materials to extend the autograft. This combination has some of the ideal characteristics of the autografts and allows the smallest amounts of autograft to go further.
ILIAC CREST BONE HARVEST
The iliac crest has been an excellent site for larger autograft bone grafting. This is an excellent site for 10 to even well over 50 cc of bone grafting material. The bone graft obtained from this site may also be incorporated with a tricortical wedge and is excellent for application in foot, ankle, and lower leg reconstructive surgery. These grafts are well incorporated as are all autografts and can be adjusted some based on the site of application need (26,28–30).

Figure 22.1 Iliac graft harvest site.
The main complication with iliac crest bone graft has been donor site pain. Other less common complications include fracture at the harvest site, infection, continued neuritic symptomatology, and invasion into surrounding structures, such as the abdomen and hip joint itself. However, it is still an excellent site with a small lateral incision directly over the anterior superior iliac spine (ASIS). Care should be taken to avoid the lateral femoral cutaneous nerve (25,31–33). Most patients have minimal donor site tenderness past the usual postoperative course (Fig. 22.1). This concurs with multiple studies, although out of all sites in the lower extremity from which the author harvests bone, this site stays particularly painful for a longer duration (25,30–36).
If a larger amount of bone is needed, harvest it from the posterior superior iliac spine (PSIS). The approach for this type of graft is more difficult and repositioning is usually needed to allow exposure and access to the reconstruction site once harvested. This harvest can be difficult in obese patients, and particular incisional care must be taken to avoid belt line pain postoperatively. Intraoperative prevention of cluneal and superior gluteal nerve damage takes specific attention to dissection technique (25,31,32). In general, the author avoids this site, as positioning and harvesting at this site has almost always doubled the anesthesia time for patients.
In the author’s experience, unless a larger amount of quality bone needs to be taken, the proximal tibia and distal tibia are far more optimal for most foot, ankle, and lower leg needs. In most practices, a separate surgeon is used for the iliac graft harvest, which may or may not be beneficial.
PROXIMAL TIBIA BONE GRAFT HARVEST
The proximal tibia at the anterior medial face of the tibia is an excellent site for bone graft harvest (12,30,35,37,38). There are minimal neurovascular structures in this region and the site could be easily and readily accessed in limited surgical time. Consistently 5 to 25 cm2 of excellent cancellous bone can be procured. This bone is of adequate quality in most patients to apply to nearly any nonstructural load-bearing region as with any other cancellous-type graft. The amount of graft harvested and present varies depending on the age and bone stock of the patient. In older patients, or with marginal bone stock, the quantity of bone in this region can be somewhat limited to 10 to 15 cc in many cases.
Figure 22.2 A. Proximal tibia bone graft harvesting site superior to the circular external fixator. B. A radiograph showing the proximal tibia donor site filled with calcium phosphate and allograft bone void filler. C. Proximal tibia harvest site filled with allograft bone chips.
The main limitation is creating a separate harvest site. The proximal tibia harvest is almost never residually painful. Other reported complications are fracture into the knee joint, infection, and harvest site pain (14,30,38,39). More common complications are a hematoma (when the cortical cap is not replaced), and also a dehiscence and hematoma when bone void filler such as calcium sulfate or calcium phosphate are introduced into the harvest site and have leaked out into the soft tissue. The other deficit of this type of harvest is a lack of true tricortical graft strut and its availability in this region. This site may be used with placement of tibia half pins and circular frames, which in theory may create a stress riser in the tibia with a potential for fracture (Fig. 22.2A), caution should be taken not to create such a riser. Bone void filler is used when taking grafts >5 cm2 (Fig. 22.2B,C).
Proximal tibia graft is most useful in applications such as pilon and/or ankle fractures, ankle fusion, excision of a more distal malunion, or specifically in the midfoot, hindfoot, or ankle reconstruction in patients with diabetes mellitus and neuropathy. It is also useful in complex nonunions. In theory, harvesting a proximal tibia graft, given its proximity to the knee, has less associated morbidity than a more distal harvest site simply because most diabetics have better blood flow at this level. This may be thought of as one less incision and wound to heal in a diabetic with already impaired distal healing potential.
Technique
Attention is directed 2 to 3 cm below the tibial tubercle on the anterior medial face of the tibia(Fig. 22.3A–F). A 5-cm incision is then carried parallel to the centerline of the tibial shaft. A he-mostat is used to spread bluntly to periosteum. Sharp periosteal dissection is made. There are minimal neurovascular structures; however, care is taken to avoid any anomalous genicular structures and the infrapatellar branch of the sphenoid nerve (which is rarely seen by the author). A small drill is used to provide uncritical stress relief to the four corners of the entrance site. Next, a small saw is used to connect the drill holes. Care is taken not to burn the bone with the saw. An astrodome may be used to finish the cuts once started. The cortical cap is lifted, which is saved for replacement latter or use distally. Round burn curettes are used to scoop upward toward the knee. The desired amount of graft is harvested. The wound is irrigated and the void may be filled with allograft bone chips, calcium phosphate, or a mixture of bone-void filler. The cortical cap is replaced. Care is taken to avoid bone-void filler or allograft from leaking into soft tissue. Meticulous periosteal closure is made, followed by irrigation and layered closure.
Figure 22.3 Proximal tibia bone graft harvest. A. Incisional site below the tibia tubercle. B. Medial tibia face exposure. C. Cortical cap and tibia predrilled corners. D. Cortical cap bone graft. E. Cancellous graft from proximal tibia. F. Postoperative radiograph showing the proximal tibia bone harvesting site.
DISTAL TIBIA BONE GRAFT
The distal tibia is an excellent source of cancellous bone (37,40–43). Many times, the cortical face or cap may be applied to the donor site in applications in midfoot fusion. This may be used in overlaying Lisfranc and Lapidus fusions to provide some structural strength and integrity (Fig. 22.4). Its size also lends to metatarsal nonunions in which the nonunited portion can be excised and the cortical cap along with cancellous underlying bone can be laid directly in the nonunited void and overlaid with fixation.
The main limitation in this region is its proximity to the ankle joint if an ankle fusion or other distal tibia or fibula type of surgery is to be performed. Other limitations are availability of bone, which is typically limited between 10 to 20 cc of cancellous bone grafting. The author has not found the cancellous quality of bone in the distal tibia medullary canal as variable in older patients with poor bone stock and osteopenia as with a proximal tibia bone graft. The other benefits of this harvest site are its proximity to the operative site, ease of the procedure, and minimal operative time.
Complications have been reported, such as fracture, entrance into the ankle joint, and entrapment of the saphenous nerve and graft site host pain, which could also include neuritis and donor site tenderness. This is an excellent site with minimal associated morbidity. It is very rare to have any significant graft harvest site tenderness from this region (30,41–43).
To help facilitate bony ingrowth similar to any significant harvest site, replace the cap over the harvest site, unless using it distally. Taking special attention and care to oversew the periosteal tissues and will almost always fill this with either calcium phosphate or a mixed calcium ceramic and bone allo-graft void filler to provide an osteoconductive bridge to regrow and fill in the medullary canal either proximal or distally in the tibia.
Figure 22.4 Application of an autologous tibial corticalcancellous cap at first met-cuneiform fusion.
Technique
Attention is directed on the anterior medial face of the tibia (Fig. 22.5A–K). The medial malleolus is felt. Four to six centimeters (cm) above this is the incision starting point. A 3- to 5-cm incision is then carried proximally parallel to the centerline of the tibial shaft. A hemostat is used to spread bluntly to the periosteum. A sharp periosteal dissection is made. There are only two neurovascular structures, the saphenous nerve and vein; they may be visualized and avoided. If the saphenous vein or branches are cut, they may be ligated. Conformation of proximity to the joint may be confirmed via fluoroscopy, but is not necessary. A small drill is used to provide unicortical stress relief to the four corners of the entrance site. Next, a small saw is used to connect the drill holes. Care is taken not to burn the bone with the saw. An osteotome may be used to finish the cuts once started. The cortical cap is lifted, which is saved for replacement later or use distally. Round burn curettes are used to scoop downward toward the ankle. Care is taken in soft or osteoporotic bone not to enter into the joint. The desired amount of graft is harvested. The wound is irrigated and the void may be filled with allograft bone chips, calcium phosphate, or a mixture of bone-void filler. The cortical cap is replaced or used distally. Care is taken to avoid bone-void filler or allograft from leaking into soft tissue. Meticulous periosteal closure is made, followed by irrigation and layered closure. Saphenous nerve entrapment is avoided; however, most often this nerve is not seen.
FIBULA
The fibula can be a fair source of bone when a cortical strut is needed. It has a limited length and amount of bone to be harvested and in many studies there is a significant graft site morbidity associated with harvesting the central third of the fibula (14,30,44,45). It is generally accepted that the distal third should be allowed to remain for adequate stability to the ankle joint. This is the area most commonly harvested for a vascularized graft. The exception would be when performing an ankle fusion or a pantalar fusion. The distal fibula is an excellent source to be used either as a ground corticocancellous graft or a partial fibula onlay strut graft. The amount of bone that can be harvested usually depends on the quality of the central medullary canal of the fibula and whether the fibula has been operated on previously (as is the case in many ankle fractures). The author typically uses the fibula as an onlay strut graft, transecting it in half and shortening it, in ankle and pantalar fusions (Fig. 22.6A,B). In ankle fusions—fixating either the proximal or distal portion, but not both—it has been the author’s experience that the remaining portions of the fibula graft are ground up within the prepared joints to help compensate for the shortening that occurs with multiple joint fusions.
Fibula grafts are limited because the quality of the graft is often poor. There is very little central cancellous bone, and these central grafts are almost all cortical material. Often when resecting the fibula as either a vascularized strut graft or onlay strut graft, the central medullary canal is very poor in cancellous stock, and the major portion of the bone itself is cortical. High donor site morbidity in this region has been reported and care must be taken to avoid injury to the peroneal tendons, sural nerve, and/or fracture at the harvest site. The author saves this particular graft harvest for only when all other choices have been exhausted or cannot be applied. The exception is fibula strut grafts for ankle and pantalar fusions and fusions of the distal tibula-fibula syndesmosis for nonunions or malunions and painfully unstable syndesmotic injuries that fail other care.
Figure 22.5 Distal tibia bone graft harvest. A. Clinical picture showing the distal tibia harvest site at the medial ankle. B. Preoperative radiograph for the tibia bone graft harvest site. C. Medial distal tibia exposure with saphenous nerve avoidance. D. Unicortical drill holes. E. Saw cut connecting drill holes. F. Utilization of a sharp osteotome to complete the saw cuts. G. Removal of the cortical cap. H. Round curette for cancellous bone graft harvest. (continued)
Figure 22.5 (Continued) I. Cancellous bone graft with cortical cap. J. Donor site is packed with an allograft bone-void filler. K. Postoperative radiograph at 1 year follow-up.
Technique
Attention is directed to the lateral 5 to 10 cm of the fibula (Fig. 22.7A). Dissection is then carried to bone. The posterior pouch of the peroneal tendons is preserved. Care is taken to avoid the sural nerve posterior and the more medial branches of the peroneal nerve above the ankle. The fibula is resected via power saw. It is then removed distally. The fibrotic tissue and distal ligament attachments are cleaned off the bone. It is then held firmly with a bone forceps and split down the middle. The remaining portions are shaped to fit the application. Use as onlay or viable graft portions may be ground.
Figure 22.6 A. Preoperative radiograph of an ankle fracture with retained hardware and post-traumatic changes and arthritis at the ankle joint. B. Postoperative radiograph showing the ankle arthrodesis with a fibula onlay strut graft.
Figure 22.7 Fibula strut graft harvest. A. Preoperative radiograph of an ankle fracture with retained hardware and post-traumatic changes and arthritis at the ankle joint. B. Fibula exposure with peroneal tendons spared. C. Saw resection of the distal fibula. D. Removal of the distal fibula. E. Transection of the fibula. F. Fibula is divided into two halves. G. Fibula onlay strut graft at the lateral aspect of the ankle fusion site. H. Proximal fixation of the fibula onlay strut graft. (continued)
Figure 22.7 (Continued) I. Postoperative radiograph showing the split fibula onlay strut graft.
CALCANEUS
The calcaneus is an excellent source of cancellous bone. It is an excellent source when needing 5 cc or less of bone and has at times taken up to 25 cc. The bone in this region is cancellous and vascular-rich and almost always seems to be of fair quality, even in the poorest bone stock. The harvest sites in this region have minimal morbidity, and the incisional tenderness tends to be no more than the surrounding surgical sites (5,12,25,30,37,38).
The main harvest sites have been described as the medial instep of the calcaneus and more often the lateral wall of the calcaneus. Incisional entrapment above the sural nerve and medial calcaneal nerve regions has been described with the above harvest sites (5,12,25,38). However, the author has found that this is usually short-lived. The other main limitation in the distal amount of blood flow in the diabetic patient leaves them another wound to heal unless incorporated into one of the other surgical site incisions. Other times harvesting graft through the calcaneus can impair surgical correction, such as in rearfoot fusions, unless used in the midfoot or forefoot application sites.
Technique
Attention is directed to the lateral wall of the calcaneus (Fig. 22.8A–G). The tip of the fibula is marked. The base of the fifth metatarsal is marked and the peroneal course is visualized. The course of the sural nerve is visualized (the lateral dorsal cutaneous nerve). A skin incision is made 2 to 3 cm inferior and parallel to the peroneal tendons on the lateral calcaneal wall. A hemostat is used to spread bluntly to the periosteum. Sharp periosteal dissection is made. A small drill is used to make a unicortical entrance site. Round curettes are used to scoop out the bone. The desired amount of graft is harvested. Care is taken not to enter the subtalar joint or opposite side calcaneal wall. A bone-void filler such as allograft bone chips or a calcium ceramic may be used at the harvest site. The periosteum is closed, followed by the skin closure. Do not close subcutaneous tissue, so as to not inadvertently entrap the sural nerve.
Figure 22.8 Calcaneal bone graft harvest. A. Intraoperative lateral view of the calcaneus demonstrating the incision placement. B. Spreading of the soft tissues to the bone level. C. Calcaneal lateral wall drilling. D. Round curette is used for the bone graft harvest. (continued)
Figure 22.8 (Continued) E. Cancellous bone graft from calcaneus used in the distal tibial fracture site. F. Donor site filled with an allograft bone-void filler. G. Postoperative radiograph showing the calcaneal lateral wall and harvest site.
The medial calcaneus may also be used as a harvest site. In this case, a medial posterior instep incision is made (Fig. 22.9A). Care must be taken to avoid the medial neurovascular bundle, and specifically the medial calcaneal nerve. This approach is useful if less bone is needed via a small 1- to 2-cm incision. The author does not fill this with bone-void filler and closes only skin (Fig. 22.9B).
OTHER SOURCES OF LOCAL BONE AUTOGRAFTS
Other sources of bone autografts include the phalangeal heads from digital joint arthroplasties, the medial eminence after a hallux valgus correction, and even portions of the navicular after a resection of an accessory or hypertrophic navicular tuberority. Sometimes it is useful to harvest hypertrophic bone formation following a Charcot foot dislocation or subluxation. Most of the usefulness of these applications is limited by the amount of bone that may be harvested. In most cases, when resecting large Charcot exostoses or the dorsal medial eminence of a hallux valgus deformity or other deformities, the bone quality is marginal, with a significant amount of soft periosteal tissue and mixed fibrous tissue. Often the bone is marginally viable from an intraoperative standpoint. However, if the fresh bleeding bone is visualized intraoperatively, this bone could be used directly into the prepared fusion sites or as an overriding onlay graft to help facilitate fusion. It is highly considered preoperatively, especially in Charcot foot and ankle reconstruction or during significant deformity correction and when the local bone is of high enough quality to be applied directly to the fusion or repair sites. The main advantage to the use of these types of bone graft is that typically they are harvested from local sites that are already being operated on or close by and have minimal associated morbidity.
ORTHOBIOLOGICS
The orthobiologics group of graft or graft type implant materials has been redefined, reinvented, and reapplied over the last 20 years. For this reason, bone grafting cannot be simply talked about in terms of autografts, allografts, and xenografts. For the purpose of this chapter, orthobiologics includes osteo-conductive materials that are bone allografts, enhanced bone allografts, calcium ceramics, including calcium phosphate, calcium sulfate, and calcium composite materials, HA products and collagen materials, including polymeric bone replacement (2,3,7,8,18,21–24,46–51).
Figure 22.9 A. Posterior medial calcaneal harvest incision. B. A postoperative radiograph showing the medial calcaneal bone graft harvest.
The use of osteoinductive materials, including the combinations of osteoconductive material mentioned, platelet gel concentrates, DBM, and BMP is also discussed (11,23,24).
There has been an explosion of different companies, manufacturers, and laboratories, and there has been an equal number of confusing mixtures of both osteoconductive and osteoinductive materials. To understand the use by application of these materials, one needs to have a working knowledge of basic properties and general applications, which will allow optimal use translating into optimal surgical results for complex reconstructive cases.
OSTEOCONDUCTIVE
Allograft
Allografts and associated alloimplant materials are excellent for most applications in foot and ankle reconstructive surgery. Many times, unless they are coupled with a significant amount of osteoinductive agent or combined with autologous bone graft, their usefulness for diabetic reconstructive surgery can be limited. Because of variable processing with allografts, they also carry some additional risk of immunologic reaction. The most common methods for processing allografts are freeze-drying, liquid nitrogen freezing, and demineralizing. The closest type of graft to one’s own bone is through the fresh-frozen technique; however, these also have the most associated immune reactions. The main advantage to fresh-frozen grafts is their retention of some osteogenic potential and properties (12,29,34,46,52).
Applications
For most diabetic foot and ankle reconstructive surgery, the additional threat posed by fresh-frozen bone grafts by an immune reaction, when other viable and predictable graft options are available, has limited the use of this particular type of grafting. The author has limited the use of fresh-frozen bone grafts for patients where a large amount of osteoconduction with osteogenic properties is needed and in which the author is hesitant to harvest this from a separate site, such as the iliac crest or tibia. These allografts have mostly consisted of tricortical fresh-frozen iliac grafts, which have been applied to midfoot and rearfoot osteotomies and some large osteomyelitic defects; however, these are some of the exceptions and not the rules. In addition, these grafts can be particularly useful when other methods are either unavailable or have failed. Most facilities will have a freezer and cold storage available for these types of grafts.
Freeze-Dried Allografts
Freeze-dried allografts, excluding DBMs, do not contain the same osteogenic potential as do bone autografts. These types of bone products are excellent for autograft bone extenders (to extend the amount of the graft harvested beyond the amount procured). They are also excellent in combination with other osteoinductive agents. Included in this is DBM independently when containing a high concentration of BMP. The main fear in the late through mid 1980s and 1990s was the transmission of disease through a same-species donor. Human immunodeficiency virus (HIV), hepatitis C, and other prions and infectious processes were feared. This created significant restrictions in the acceptance of donors and also standardized guidelines for tissue banks. This has all but eliminated the fear for most surgeons and in most patients. In almost all cases, the benefit of graft application outweighs the extremely low likelihood of any disease transmission or antigenic reaction (12,29,34,46,52,53).
Applications
Freeze-dried allograft has mainly been used during reconstructive surgery for large bone voids. The author has found it particularly useful in filling the graft harvest site in the distal or proximal tibia or even the calcaneus. In large resolved osteomyelitic voids or nonunions, freeze-dried allograft in combination with DBM gel or autograft is sometimes used. Rarely, unless no other choice remains, freeze-dried allograft is used alone across a fusion site or a revision fusion site in which bony union across the site is anticipated and needed for the reconstruction. Large fracture voids may be filled with cancellous allograft in vascular areas of bone (Fig. 22.10). Lateral column procedures such as the Evans calcaneal osteotomy are also good application sites for tricortical iliac crest allografts (Fig. 22.11A–C).
Figure 22.10 Allograft cancellous bone chips for calcaneal fracture void.
BONE FIXATION DEVICES
Bone fixation devices have found some usefulness in reconstructive surgery. Difficult fusion and revision fusions of toes and even larger screw fixation and pin fixation devices made from cortical bone have been available on the market. Their minimal antigenicity has made them an option for some reconstructive surgeries. The main limitations have been their variable strengths, slow incorporation, and lack of strength when compared with standard rigid internal fixation. The benefit of these devices has mainly been the lack of need for removal, density, and strength equal to the bone that they are being placed into and their general availability (11,23,24,34,46,53–55).
Figure 22.11 A. Tricortical iliac crest allograft for Evans calcaneal osteotomy. B. Allograft DBM putty applied around the tricortical iliac crest allograft and within the osteotomy site. C. Internal fixation at the surgical site with the tricortical iliac crest allograft and DBM putty.
The author has found limited use in diabetic reconstructive surgery, especially major reconstructive cases in which other options have shown clinically repeated excellent results such as standard internal/external fixation or adjuncts of both. This will continue to be an emerging market in which great expectations may lay in the future.
Hydroxyapatite
Hydroxyapatite (HA) grafts are considered xenografts, as they are comprised of Gonioptera coral species. These grafts are purely osteoconductive bridges that may be used to fill minimally load-bearing or protected voids in bone. HA come in a 200- and 500-mcg pore size. This approximately mimics cortical and cancellous bone, respectively (ProOsteon 200R and 500R, Interpore Cross, Irvine, CA). The structure of HA makes it excellent for large bone voids and cysts—and its use may be widened when mixed with a DBM autograft or osteoinductive agent.
Figure 22.12 A. Preoperative radiographs of a diabetic neuropathic distal tibia malunion. B. Preoperative clinical picture of the distal tibia varus malunion. C. Intraoperative distal tibia osteotomy and deformity correction. D. HA bone graft wedge with allograft bone cancellous chips and DBM paste. E,F. Postoperative radiographs. G. Postoperative clinical picture after correction.
HA is useful as a building block or structure to add to, in large voids after pilon fractures, severe calcaneal void fractures, and after resecting large tibia defects (Fig. 22.12A–G). It is also good for filling the void at the majority of autograft harvest sites. At most sites, the application of HA must be rigidly protected by both surrounding bone and fixation. The slow osteoclastic breakdown of HA prevents any significant usefulness at fusion sites (8,12,15,47,56–61).
CALCIUM CERAMICS
Calcium Phosphates
Calcium phosphates have use in their various forms in filling large voids. The main drawback of calcium phosphate, as with other ceramics, is that they are clearly osteoconductive.
These are best used to fill large bone voids and autogenous harvest sites; however, when applied into larger defects, the author prefers to do this in deeper tissue where the periosteum can be oversewn or a cortical cap can be placed. It is also useful in tumor voids when mixed with allograft (Fig. 22.13). The other drawback with calcium phosphate is that when it does get into soft tissue it can create a soft tissue sterile abscess that can go on to dehiscence. The author has seen this many times when not careful and has gotten some of the pellets or paste into the soft tissue. There have also been studies in which calcium sul-fate mixtures have been useful in calcaneal fracture repairs, allowing early weight-bearing and mobilization (62). In the author’s own practice, it was not found to benefit most calcaneal fractures, fusion, or reconstructive surgeries outside of the noted purpose for large applications of calcium phosphate or calcium sulfate paste (8,16,18,19,23,51,56,61,63–70).
Calcium Sulfate
Calcium sulfate does come in many forms. The most common are pellets. These also have been very useful in filling large bone voids, large defects, and donor sites in the proximal distal tibia and pelvis. These, like calcium phosphates, are even worse when they leak into the soft tissue or are left in the soft tissue, as they seem to melt and have spontaneous sterile abscess within the site itself. The author rarely if ever uses any form of calcium sul-fate, calcium phosphate, or other ceramic material at any fusion site or other site where cyclical loading and bone repair would be expected to occur to any reasonable extent beyond the conductive bridge they provide (11,16,18,23,24,50,71,72).
Figure 22.13 Mixed calcium ceramic paste injected into first metatarsal tumor void.
Applications
The author has used antibiotic-impregnated calcium sulfate in large soft tissue defects with underlying osteomyelitis, but tends to avoid leaving these in any soft tissue or periosteal regions where then can possibly leak out given their tendency to discharge and dehisce at the wound sites, and thus create a non-healing environment. They have been applied to large defects after resection of osteomyelitis; however, the author does not rely on them to rid the bone of osteomyelitis or bridge or span the bone to facilitate bone healing. These peoperties are usually indicated for autogenous or another bone allograft mixture in combination with other bone growth factors or BMP. Common forms include OsteoSet pellets (Wright Medical Inc, Arlington, TN), Jax (Smith & Nephew Inc, Memphis, TN), calcium sulfate, and many other variances (Fig. 22.14A,B).

Figure 22.14 A. Distal tibia harvest site. B. Calcium sulfate applied to distal tibia harvest site to fill void.
COLLAGEN MATERIALS
Collagen has been combined with multiple other materials to help create a better bone graft supplement. Collagen is an excellent carrier of other materials, including calcium phosphate and calcium sulfate, but its use seems more applicable to irregular defects since it may be shaped, cut, and packed into a variety of situations, especially when combined with an osteoinductive material (11,18,23,24,48,51,73).
Applications
The main applications in reconstructive surgery have been as bone graft extenders, packing bone voids, and non-weight-bearing structural defects of the tibia and calcaneus. They have been used for supplementary overlay grafts in addition to autogenous bone grafts, but they are still combined with DBM or other osteoinductive materials. Two common and available agents on the market today are Healos (DePuy, Warsaw, IN) and Collagraft (Zimmer, Warsaw, IN). Healos is a bovine collagen and HA material. Collagraft is HA and tricalcium phosphate mixture.
OSTEOINDUCTIVE AGENTS AND MATERIALS
There are a variety of osteoinductive agents. This chapter includes DBM, platelet gel concentrates, BMP, and MSCs. Marshall Urist’s isolation of BMP in the 1960s led to advancements in isolated growth factors and other osteoinductive agents. Over the last decade research and technology have caught up to one another to allow an explosion in the number of materials available for surgical use. This will continue to be a merging market, as further biological stimulant and growth factors are isolated through progressive research (2–4,6,10,11,13,21,22,24,49,74,75,77-80).
Demineralized Bone Matrix
DBM has been used for many years, since residual properties of bone morphogenetic proteins were originally discovered. In addition to BMP, other growth factors continue to be isolated, and many of these have been shown to be beneficial in bone healing. These include insulin-like growth factor, osteocalcin, osteonectin, and transforming growth factor beta. Most of the carriers with DBM are a glycerol-type gel similar to some of the delivery systems with calcium pellet products. Most of the DBM products vary only in the concentration of protein gels or BMPs (2,6,24,81–83).
DBM is a mixture of cortical and cancellous bone organic material. Because of the different techniques and processing of DBM it is likely and significant that many products contain variable amounts of different types of BMP. The benefit and ideal properties of various BMPs described are not fully known or realized; however, it is generally accepted that almost all BMPs create a rich environment for osteoinduction, which is beneficial for bone healing (6,18,24,82,83).
Applications
DBM is very useful across fusions and nonunions, and in Charcot reconstruction. These have been found both in gel and paste bases to be very useful at fusion and fracture sites to fill in or smooth out small residual voids and irregularities before fixation is added. It is easily taken off the shelf and applied as a primary graft or more commonly in combination with allo-graft bone.
DBM is readily available and may also be added to combine with calcium ceramic and other materials to give them some osteoinductive capabilities. DBM has been proved in patients undergoing complex arthrodesis and nonunions to be compatible to autograft. They have been found particularly useful in simple fusions in the forefoot and midfoot, and more complex in the rearfoot and ankle as well as nonunions of the tibia, fibula, and foot. They are particularly useful in Charcot reconstruction applications and deformity corrections. The author does not use them alone, but most often mixes them with an autogenous bone graft. The main disadvantage is their lack of structural integrity; however, the author finds DBM the most useful of all the osteoinductive agents on a regular consistent basis (Fig. 22.15).
Platelet Gel Concentrate
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree