Introduction
Water and volume homeostasis are under meticulous control through a complex interrelationship of the hypothalamus–posterior pituitary and the renin–angiotensin–adrenal axis.1 The elderly, however, are at increased risk for both syndromes of hyponatraemia and hypernatraemia and these disorders are associated with further clinical complications.2–4 It is therefore important to understand the physiology involved in normal water homeostasis, the potential problems associated with ageing and the possible therapeutic modalities to correct these disorders.
Normal Physiology
There is dual control of water and serum osmolality.1 The hypothalamus and posterior pituitary are involved in water retention and the renin–angiotensin–aldosterone axis is involved in sodium retention.
The supraoptic and paraventricular nuclei of the hypothalamus respond primarily to increases in serum osmolality with the release of vasopressin [antidiuretic hormone (ADH)] Figure 96.1a. The release of ADH is mediated through changes in an electrochemical gradient in these magnocellular neurons to maintain serum osmolality at 285 ± 2 mOsm kg−1. These neurons are also under the influence of neurotransmitters, such as acetylcholine (i.e. through the vagus nerve, as described below), catecholamines, opioids and angiotensin. Small changes in osmolality allow for acute adjustment of serum ADH levels with resulting water retention or free water clearance in the kidney.1
Figure 96.1 (a) Osmotic control of water balance. The hypothalamus supraoptic (SO) and paraventricular (PV) nuclei release antidiuretic hormone (ADH) through neural tracts to the posterior pituitary. Thirst is under control of a closely located series of hypothalamic neurons in the organum vasculosum of the lamina terminalis (OVLT). (b) Pressure–volume control of water and sodium balance. Baroreceptors (systemic blood pressure) in the aortic arch and volume receptors in the atria respond to changes in effective arterial blood volume (EABV) to induce release of ADH through vagal stimuli. Decreased intrarenal perfusion induces renin activation of the renin–angiotensin-aldosterone system to increase sodium retention.
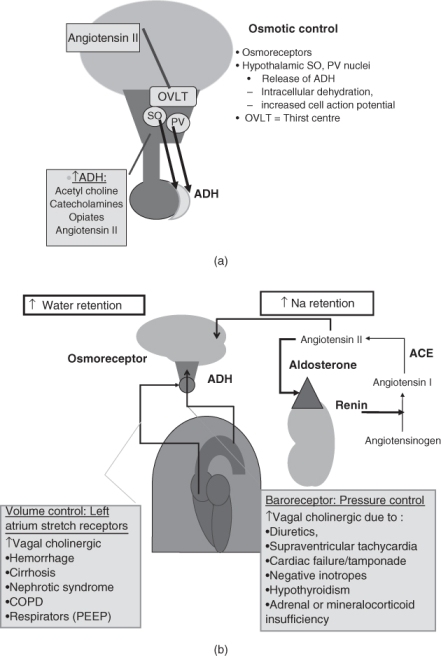
There is also a parallel autonomic nervous system regulation of water retention in which vascular receptors respond to decreases in total body water and changes in organ perfusion. There are high-pressure (blood pressure) baroreceptors in the carotid sinus and aortic arch and low-pressure stretch receptors in the cardiac atria and pulmonary venous systems. Both types of receptors transmit regulatory impulses via the vagus nerve to the hypothalamic neurons to stimulate ADH in the event of low effective arterial blood volume (EABV).1 Pathophysiological states of diminished EABV will stimulate the release of ADH above that due to osmolality. Thus ADH may be ‘appropriate’ for the diminished EABV, but appears inappropriate for serum osmolality. Conditions which may increase ADH release are shown in Figure 96.1b. The baroreceptors may respond to changes in blood pressure associated with volume loss (gastrointestinal losses or diuretic-induced volume loss), decreased intravascular volume in hypoalbuminaemic oedema-forming states (ascites or nephrotic syndrome), orthostatic hypotension (due to adrenal cortical insufficiency, mineralocorticoid insufficiency or autonomic neuropathy) and decreased arterial perfusion (due to reduced cardiac output such as cardiac tamponade, cardiomyopathy or severe hypothyroidism). Decreased stretch of the volume receptors in the cardiac left atrium may occur in states of low EABV as described above. Diminished stretch in these receptors may also ‘appear’ as low pressure due to restrictions in pulmonary vascular return to the heart, as a result of increased intra-thoracic pulmonary pressure in severe reactive airway disease or with mechanical ventilation.1
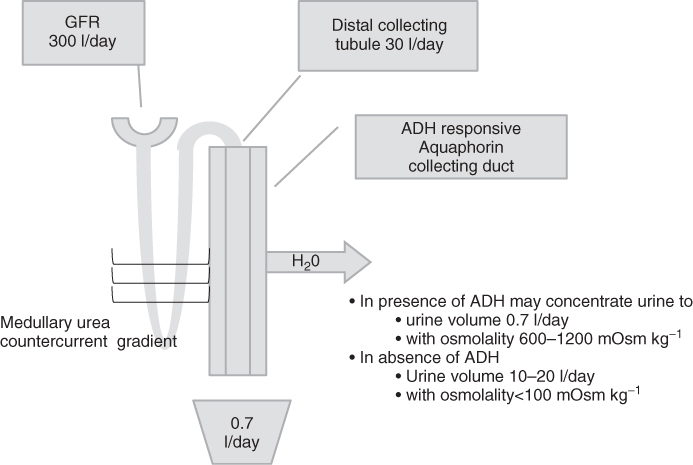
Vasopressin activates V2 receptors in the distal collecting tubule of the kidney (Figure 96.2). In the absence of ADH, the tubule is impermeable to water transport. As shown in Figure 96.2, 15–30 l per day of free water may reach the distal collecting tubule. The entire volume may potentially be lost through the urine in central diabetes insipidus (lack of renal concentration ability due to either partial or complete ADH deficiency). In the presence of ADH, water is transported from the intraluminal collecting duct through aquaphorin-2 channels, across a concentration gradient to the intra-renal capillaries to reabsorb free water. The concentration gradient is derived at the loop of Henle through the medullary urea countercurrent system. The urea is freely permeable into the collecting duct. In the presence of ADH, the kidney may concentrate the urine to a volume of 0.7 l per day with an osmolality of 600–1200 mOsm kg−1, made up primarily of the secreted urea.
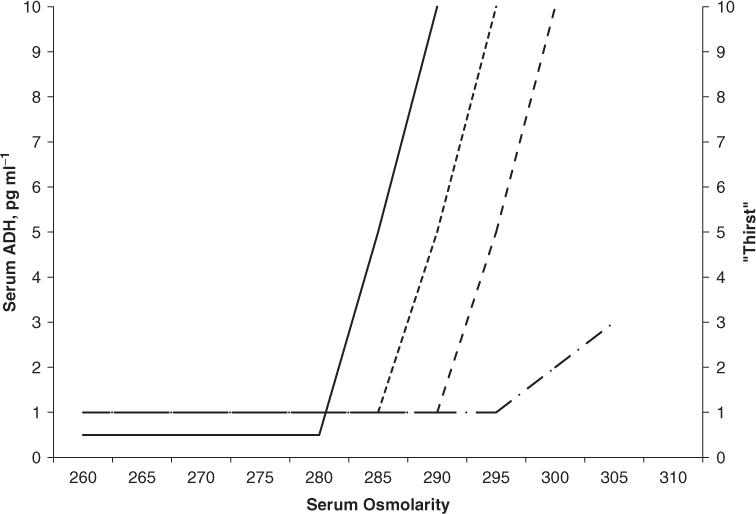
Thirst, the conscious desire to drink, is another active component of water retention.1, 5 Thirst is under control of a closely located series of hypothalamic neurons in the organum vasculosum of the lamina terminalis (OVLT). This area is independent of the blood–brain barrier. These neurons, like the ADH-secreting neurons, are under a similar influence of serum osmolality, neurotransmitters and angiotensin. Normally thirst lags ADH release in response to increases in serum osmolality. The threshold of thirst, as measured on a visual analogue scale or the volume of water ingested, is ∼10 mOsm kg−1 greater than the ADH threshold.5 There are pathological conditions in which thirst may be independent of ADH release. Thirst is inappropriately diminished in response to serum osmolality in central nervous system conditions characterized by a reset or diminished thirst response to serum osmolality (essential hypernatraemia) or in complete lack of thirst response to severe hypernatraemia (adipsia) (Figure 96.3).5
Figure 96.3 Comparisons of disorders of thirst. Serum ADH increases with serum osmolality at a threshold of 280 mOsm kg−1 (solid line). Thirst responses increase at approximately 5–10 mOsm kg−1 higher than that of ADH release (dotted line). Abnormally decreased thirst responses are found in essential hypernatreamia (dashed line) and severely impeded thirst responses are found in adipsia (dashed-dotted line).
Whereas ADH is the main hormone involved in water homeostasis, the renin–angiotensin–aldosterone system is the main factor in sodium retention and systemic blood pressure/volume control. Renin is released from the juxtaglomerular apparatus of the kidney in response to low perfusion, low intravascular volume and low tubular sodium. Renin is an enzyme which converts liver-derived angiotensinogen to angiotensin 1 and lung-derived angiotensin-converting enzyme further metabolizes conversion to angiotensin 2. Angiotensin 2 both stimulates the release of the mineralocorticoid aldosterone to retain sodium and stimulates the hypothalamic neurons to release ADH and provoke thirst (Figure 96.1b).
Syndromes of hyponatraemia may reflect physiological ‘appropriate’ release of ADH in response to vagal stimuli due to decreased perfusion pressure or decreased plasma volume (decreased EABV). In these situations, the elevated ADH is inappropriate for the serum osmolality. The syndrome of inappropriate ADH secretion (SIADH) is a research definition which eliminates appropriate physiological ADH responses. The discussion of hyponatraemia includes both ‘appropriate’ and SIADH syndromes.
Hyponatraemia may be defined as a serum sodium level <135 mequiv l−1 and for clinically significant hyponatraemia <130 mequiv l−1.1 Aside from water intoxication associated with excessive water intake during exercise, most causes of hyponatraemia are associated with imbalances in ADH levels.2–4, 6 Clinical syndromes of hyponatraemia (and true hypo-osmolality) are associated with decreased effective serum osmolality,7, 8 where
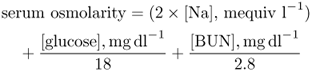
Serum concentrations of urea, which are included in the calculation of plasma osmolality, are not considered as part of the calculation of effective extracellular osmolality, since urea is freely permeable through cell membranes.
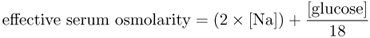
Therefore, the major component of extracellular osmolality in the non-hyperglycaemic state is serum sodium with its corresponding anions. Severe clinically significant hyponatraemia is usually associated with serum sodium in the range <120 mequiv l−1 or with the rapid decline of serum sodium as in water intoxication or post-anaesthesiology hyponatraemia.1, 6 The major toxicities are due to changes in neurological functions (defined as hyponatraemic encephalopathy).1, 6 Symptoms may range from headache, nausea, disorientation and confusion to more severe symptoms of cerebral oedema with seizures, coma and, in extreme cases, cerebral tentorial herniation and death. Chronic hyponatraemia (which has developed over a time course of >48 h) usually results in central nervous system intracellular adaptation, with the extrusion of intra-neuronal organic and inorganic osmoles. During the treatment of symptomatic hyponatraemia, the concern therefore is that overly rapid correction of hyponatraemia (defined as an increase of >12 mequiv l−1 over 24 h or >18 mequiv l−1 over 48 h) may result in cerebral dehydration and pontine and extrapontine osmotic demyelination syndromes (ODS). These ODS may be delayed in onset and associated with severe neurological morbidity and mortality.1, 6
Water Homeostasis in the Elderly
The elderly are prone to disorders of both hyper- and hyponatraemia.1–6 These abnormalities may be due to the normal physiological changes in the ageing process, intercurrent illnesses or side effects of medications. Normal physiological changes due to ageing may result in a tendency towards hypernatraemia (sodium >145 mequiv l−1).4, 5, 9 Although renal function declines with age, fluid homeostasis is not affected by this decline until glomerular filtration rates are as low as 30–50 ml min−1.4 Compared with younger individuals subjected to water deprivation, healthy older adults have decreased thirst responses and increased serum ADH levels, but decreased urinary concentration and ability to excrete free water.10, 11 The decreased responsiveness of aquaporin-2 to ADH may be due to a physiological decreased aquaporin-2 receptor expression associated with ageing.12 Also, after age 75 years there is a decrease in total body water from 60 to 50%, potentiating the risk for dehydration over short periods of time.4
The institutionalized elderly may be more prone to hypernatraemia.9, 13 Whereas normal elderly patients subjected to fluid restriction may have a decrease in thirst response compared with younger subjects, they retain their ability to secrete ADH. Those with Alzheimer’s disease may be more severely compromised by having a more pronounced decrease in both thirst and ADH responses compared with even age-matched controls.14 In patients with Alzheimer’s disease, these thirst responses may fall in the range compatible with essential hypernatraemia5 and the ADH levels may be inappropriately low for the degree of dehydration and comparable to levels found in states of partial central diabetes insipidus.14 Unless patients are monitored, elderly institutionalized individuals dependent on caregivers for fluid intake (due to previous stroke or degenerative brain diseases) may not receive adequate fluids at or between meals.13 These groups of people may have an 18% incidence of hypernatraemia.5 The incidence of hypernatraemia may be exacerbated during acute intercurrent febrile upper respiratory illness to levels as high as 63%.9 They may also have concurrent illnesses (such as hypercalcaemia or hypokalaemia) or be prescribed medications (such as lithium), all of which are associated with nephrogenic diabetes insipidus (renal insensitivity to ADH action) and inability to retain water.
Hyponatraemia is also very common in the elderly, as outpatients, inpatients and those in long-term care.15, 16 The prevalence of hyponatraemia among elderly outpatients is 7–11%4 and for those in long-term care 11–53%.2, 3, 15 The causes of hyponatraemia are less clearly defined than for hypernatraemia. There are physiological changes in the kidneys with ageing resulting in a decreased ability to concentrate urine and to excrete free water.11, 17 However, the onset of hyponatraemia may be associated with medication or responses to concomitant illnesses.15, 16 Many medications involved in central nervous system modulation and opioid transmission are associated with ADH secretion. Common agents associated with hyponatraemia in all people include antidepressants (both tri- and tetracyclics), antipsychotic drugs (phenothiazines, butyrophenones), antiepileptic drugs (carbamazepine, oxcarbazepine, sodium valproate) and opioids.18 The elderly appear to be more sensitive to the hyponatraemic effects of selective serotonin reuptake inhibitors (SSRIs).19 Diuretics, owing to their frequent use, are probably the most common medication associated with hyponatraemia, with a prevalence as high as 11% in the geriatric population.18 Less commonly, other antihypertensive agents such as angiotensin-converting enzyme inhibitors and calcium channel antagonists produce a decrease in effective arterial blood volume (EABV) with physiologically ‘appropriate’ increases in ADH.18 True SIADH syndromes, due to ectopic ADH production by cancer or inappropriate ADH due to neurological lesions, are less likely to be the cause of hyponatraemia unless there are positive clinical features. Among 50 elderly hospitalized patients with mild to moderate hyponatraemia, an exhaustive evaluation did not reveal these causes of inappropriate ADH syndrome. The investigators found that the hyponatraemia was associated with pneumonia and medication, although 60% remained idiopathic.16
Many patients may have primary orthostatic hypotension, for example, due to autonomic neuropathy in Parkinson’s disease and multiple system atrophy or associated with low renin–low aldosterone mineralocorticoid deficiency. Older patients may have excessive treatment of their hypertension. The elderly should be monitored for orthostatic blood pressure changes and have more moderate adjustment of systolic hypertension than younger individuals.20
Other medications, not specifically used in the elderly, are associated with hyponatraemia. Antineoplastic agents include vincristine and cyclophosphamide. Vincristine may cause a hypothalamic neuropathy and cyclophosphamide treatment may potentiate an ADH effect at the renal tubule and requires patients to drink large volumes of water to prevent cystitis.18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree