Thrombosis is a common complication of cancer, occurring in up to 15% of patients. This article reviews the diagnosis and management of the most common cancer-related thrombotic problems; deep venous thrombosis, pulmonary embolism, and catheter-related thrombosis. Rarer entities, such as cerebral vein thrombosis and Budd-Chiari syndrome, are also reviewed.
Pulmonary embolism/deep venous thrombosis
Natural History
It is estimated that pulmonary emboli occur in at least 0.5 to 1 per 1000 people in the United States per year, leading to at least 50,000 to 100,000 deaths. More than 90% of pulmonary emboli occur as a complication of thrombosis in the deep venous system of the legs. Therefore, treatment and prevention of deep venous thrombosis (DVT) will reduce the occurrence of pulmonary embolism (PE). Another key point is that more than 90% of the deaths from PE occur within the first hour. Thus, management is aimed at prevention of a repeat embolism and not treatment of the initial embolus. Every aspect of this risk is magnified in patients with cancer, because they are more likely to have thrombosis, more likely to die of their thrombosis, and more likely to have complications of antithrombotic therapy.
Pathophysiology
Cancer patients may develop thrombosis for multiple reasons : in some, the tumor itself expresses procoagulant proteins, such as tissue factor, which directly activate coagulation; in some, large, bulky tumors, such as lymphoma, can cause obstruction of the venous system; and many have high levels of inflammatory cytokines that can directly activate the coagulation system.
Another major etiology of cancer-related thrombosis is therapy. The presence of cancer triples the risk of thrombosis in any surgery. It is estimated that in brain surgery for malignant tumors, as many as 60% of patients will develop thrombosis. This increased risk of thrombosis can be present for up to 6 weeks after surgery.
Chemotherapy can also increase the risk of thrombosis. Early studies showed that receiving adjuvant therapy for breast cancer resulted in a 6.5-fold increase in thrombosis. The newer angiogenesis inhibitors, such as thalidomide, sunitinib, and bevacizumab, have marked thrombotic risks. For example, without prophylaxis, 25% of patients receiving thalidomide and chemotherapy developed thrombosis. Finally, all forms of hormonal therapy of breast cancer are associated with a 2- to 3-fold increased risk of thrombosis.
Diagnostic Tests
Patients most often first notice dyspnea and cough after a PE. Chest pain occurs hours to days after the event, with the development of lung infarction. Less than one-third of patients will have hemoptysis, and 10% to 20% will have syncope. Most patients, on examination, will have tachypnea (70%–92%), but less than half have tachycardia. Chest radiographs are normal in only 30% of patients with PE. A nonspecific infiltrate is seen in 50% to 70% of patients with PE and an effusion in 35% of patients with PE. In recent studies, 15% to 30% of patients had partial pressure of oxygen (P o 2 ) greater than 90 mm Hg, and 20% to 30% had alveolar-arterial gradients less than 20 mm Hg. These results demonstrate that patients with PE need not be hypoxic or have an abnormal a-A gradient.
Recently, there has been great interest in clinical prediction rules for DVT and PE. Using these rules, clinicians can better predict which patients are at a higher risk for thrombosis. Validated rules for DVT and PE are summarized in Tables 1 and 2 . Of note, active cancer is an important component of each of these rules. Use of these prediction rules helps risk-stratify patients and aids in determining the sequence of diagnostic tests.
| Variable | Points |
|---|---|
| Active cancer | +1 |
| Paralysis or recent plaster immobilization of lower extremity | +1 |
| Recently bedridden for >3 d or major surgery within 4 wk | +1 |
| Local tenderness CM | +1 |
| Calf swelling >3 cm than asymptomatic side (measured 10 cm below tibial tuberosity) | +1 |
| Pitting edema in symptomatic leg | +1 |
| Dilated superficial veins (nonvaricose) in symptomatic leg only | +1 |
| Alternative diagnoses as or more likely than DVT | −2 |
| Wells: Variable | Points |
|---|---|
| Clinical signs and symptoms of DVT | +3 |
| PE as likely or more likely than alternative diagnosis | +3 |
| Immobilization or surgery in past 4 wk | 1.5 |
| Previous PE or DVT | 1.5 |
| Heart rate more than 100 beats/min | 1.5 |
| Hemoptysis | 1 |
| Active cancer | 1 |
| Low probability, <2; intermediate probability, 2–6; high probability, >6. | |
| Geneva: Variable | Points |
|---|---|
| Previous DVT or PE | +1 |
| Recent surgery | +1 |
| Age >65 y | +1 |
| Cancer | +1 |
| Unilateral lower limb pain | +1 |
| Hemoptysis | +1 |
| Heart rate (beats/min) | |
| 75–94 | +1 |
| ≥95 | +1 |
| Pain on lower limb palpation and unilateral edema | +1 |
| Probability: low, 0–4; intermediate, 5–8; high, >9. | |
| Probability of PE | % of Total Patients | % with PE | ||
|---|---|---|---|---|
| Wells | Geneva | Wells | Geneva | |
| Low | 57 | 36 | 3.6–7.1 | 7.7 |
| Medium | 36 | 60 | 18–25 | 29.4 |
| High | 7 | 4 | 50–66 | 64.3 |
A major advance in the evaluation of patients with DVT/PE is the wide availability of rapid D-dimer assays. Thrombi have areas that are growing and other areas that are undergoing fibrinolysis. One of the breakdown products of thrombi is called a “D-dimer” whose levels reflect the thrombus burden. All patients with clinically significant thrombosis will have levels of D-dimers above the assay cutoff, thus making it a sensitive screening test for thrombosis. Confusion arises because there are three different types of D-dimer assays available, all with different abilities to help in diagnosing DVT/PE.
- •
Latex agglutinin—used for the diagnosis of disseminated intravascular coagulation and usually reported as a titer (for example, “2–4”); lacks sufficient sensitivity to be used as a test in thrombosis.
- •
Point-of-care D-dimer test—offers binary “yes-no” results ; has higher sensitivity, but must be used with decision rules. Most studies show that the combination of a negative point-of-care D-dimer test and a low-probability result on a prediction rule is sufficient to rule out thrombosis without the need for imaging.
- •
“High-sensitivity” D-dimer test—sensitivity approaches 95%. Combination of a negative D-dimer test and a low-probability result on a prediction rule is sufficient to rule out thrombosis without the need for imaging.
One drawback of the D-dimer test is its lack of specificity, coupled with its high sensitivity. Therefore, patients with positive D-dimer assays require further testing to establish the presence of thrombosis. Patients with recent trauma, recent surgery, pregnancy, or who are older than 70 years have a higher baseline D-dimer level, which greatly limits the use of D-dimers in these patients. Cancer patients also have a higher incidence of increased D-dimer levels; however, there are still a reasonable percentage of patients with negative D-dimers (9%–15%), which makes the test useful for screening patients.
Currently the standard for definite diagnosis of PE is CT angiography (CTA) of the chest. Diagnostic approaches that use only CTA have excellent outcomes when compared with those that combine CTA with leg studies or other imaging modalities. Sensitivity and specificity are higher for embolism in the segmental and larger blood vessels. Controversy continues over the clinical implications of isolated subsegmental PE because of this being a common finding and the lack of specificity of this finding. A growing concern regarding CTA is the potential overuse of this test and exposure to unnecessary radiation. In many institutions, the positive rate of CTA for embolism is only 5% to 10%. Radiation exposure from a CTA can be equivalent to 100 to 400 chest radiograms or 10 to 30 mammograms, which highlights the need for a structured approach to PE diagnosis.
One issue that has occurred since the widespread use of CTA is the finding of an “unexpected” PE on a CT scan done for another reason, such as cancer screening. In retrospect, many of these patients were having symptoms, such as increased dyspnea. Patients with unexpected PE on CT have increased mortality and need to be treated as aggressively as any patient with PE.
Ventilation perfusion scans are sensitive but not specific for PE. The use of these tests has declined dramatically in the past decade, making it difficult to readily obtain these tests and raising concerns about reliability of interpretation. Only a normal scan rules out embolism, and positive scans have to be interpreted with the patient’s pretest probability of thrombosis along with the pattern of the scan. Pulmonary angiography is the gold standard for diagnosis of PE but is rarely performed in the modern era.
Doppler ultrasound is the definitive diagnostic test in patients with symptoms of DVT. Sensitivity and specificity are greater than 95% for lower-extremity thrombosis. Use of venography or CT may be appropriate in patients with a high suspicion of thrombosis but with a negative ultrasound.
Diagnostic pathways
Deep venous thrombosis
Pretest probability should be determined using the Wells rule. If a high probability of DVT is not present, then the emergency physician should obtain a high-sensitivity D-dimer test. If the D-dimer level is normal, an alternative diagnosis needs to be considered, and no further testing is needed for thrombosis. If the patient has a high probability score or positive D-dimer test, then a Doppler examination is performed. If the Doppler is positive for thrombosis, anticoagulation therapy should be started immediately in the emergency department (ED) ( Fig. 1 ).
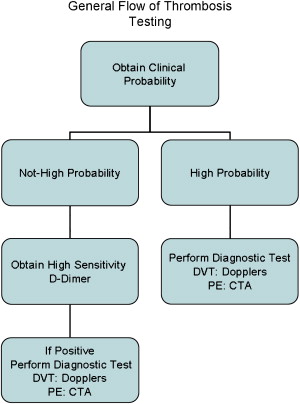
Pulmonary embolism
Pretest probability should be determined using the Wells or Geneva rules. If a high probability of DVT is not present, then the emergency physician should obtain a high-sensitivity D-dimer. If the D-dimer is normal, an alternative diagnosis needs to be considered, and no further testing is needed for thrombosis. If the patient has a high-probability score or positive D-dimer, then a CTA is performed and, if positive, anticoagulation therapy is started in the ED.
Immediate Therapy of Thrombosis
Thrombolytic therapy
Given the natural history of PE, the role of thrombolytic therapy is uncertain. The fact that thrombolytic therapy lyses clots faster than heparin seems to be of no long-term clinical significance in most patients. Even in patients with PE associated with right ventricular dysfunction, use of thrombolytic therapy failed to show an improvement in death rates. Many patients with PE are poor candidates for thrombolytic therapy due to recent surgery or other reasons. Also of concern is the 1% to 2% risk of intracranial hemorrhage that accompanies thrombolytic therapy. The vast majority of patients with PE who survive long enough to be diagnosed with their embolism will probably survive the acute event. Their increased delayed mortality with PE is mainly due to underlying diseases, such as cancer. Therefore, only a small number of patients would benefit from thrombolytic therapy. However, fibrinolytic therapy remains an option for the patient in extremis because of a PE who is not a candidate for embolectomy ( Box 1 ).
Immediate therapy
Thrombolytic therapy
Pulmonary embolism: consider in patients with refractory hypotension
Deep venous thrombosis: consider catheter-directed therapy for iliofemoral thrombosis
Embolectomy: consider in PE patients with cardiac arrest or refractory hypotension
Inferior vena cava filters: consider if patients have a contraindication to anticoagulation therapy
Heparin
Heparin: bolus 5000 to 10,000 units followed by 1000 to 2000 units/h to achieve heparin levels of 0.35 to 0.7 anti-Xa units
Low–molecular weight heparin:
Dalteparin: 100 units/kg every 12 h
Enoxaparin: 1 mg/kg every 12 h or 1.5 mg/kg in low-risk patients
Tinzaparin: 175 units every 24 h
Fondaparinux (pentasaccharide): 7.5 mg every 24 h (5.0 mg in patients weighing less than 50 kg and 10 mg in patients weighing more than 100 kg)
If thrombolytic therapy is required, the dosing for the medications is the same as that for cardiac indications. Plasma fibrinogen and activated partial thromboplastin time (aPTT) should be measured every 4 hours after treatment. If the aPTT is below two times normal and the fibrinogen is more than 100 mg/dL, heparin should be started.
Intravenous thrombolytic therapy for DVT has little effect on long-term outcomes, such as post-phlebitic syndrome. It therefore has little role in the management of these patients. One area where thrombolytic therapy is increasingly useful is when using catheter-guided lytic therapy to recanalize the vein in massive DVT, involving the common femoral or iliac system. Often, these patients with underlying venous compression can also have venous stenting or vasoplasty to fix the lesions during catheterization.
Surgical embolectomy may be useful in the small subset of patients who are in unresponsive shock. Some series claim up to 70% survival. A patient may be a candidate for embolectomy if persistent signs of shock remain with a suspected or confirmed massive PE after an hour of medical management in the ED setting. Specific examples of persistent shock in adult patients would include a systolic blood pressure of less than 90 mm Hg, urine output of less than 20 mL/h, or P o 2 of less than 60 mm Hg. This approach requires the presence of a qualified cardiothoracic surgeon.
The role of inferior vena cava filters in the treatment of thromboembolic disease is unclear because of a lack of good trials. A strong indication for filter placement would be PE with DVT in a patient in whom anticoagulant therapy is transiently contraindicated. However, a filter is never a replacement for long-term anticoagulation therapy, so even in these patients, anticoagulation therapy needs to be started as soon as feasible. This is especially true in cancer patients; 17% to 66% of those who receive filters instead of anticoagulation therapy will have thrombotic complications.
Elastic compression stockings are extremely useful in the prevention of post-phlebitic syndrome. All patients with DVT should be prescribed knee-high stockings with the compression “dose” being 30 to 40 mm Hg at the ankles. Patients should be advised to wear stockings most of the day and everyday for best effect.
There is now abundant evidence that using low–molecular weight heparin (LMWH) for therapy in DVT and PE treatment is just as effective as unfractionated heparin for any type of venous thrombosis, ranging from submassive PE to superficial thrombophlebitis. There are also data supporting the use of pentasaccharide fondaparinux; however, its long half-life and renal clearance may be concerns in older patients or those with renal problems.
For most patients receiving LMWH therapy, laboratory monitoring is not required. Monitoring should be considered for patients who are very obese (greater than 2 times their ideal body weight), who have severe liver or heart failure, who are pregnant, or who require long-term therapy. Very obese patients still require actual body weight–based dosing without “capping” the dose at an arbitrary level but require a level the second day of therapy. LMWH is cleared by a renal mechanism and will accumulate in patients with renal failure. Therefore, in patients with renal failure, initial dosing should be reduced by 50% and levels monitored. However, the use of LMWH is not associated with an additional risk of bleeding when compared with standard heparin. All patients should receive at least 5 days of heparin therapy. Recommended initial LMWH doses are summarized in Box 2 .
Antiphospholipid antibodies
Behçet syndrome
Myeloproliferative syndrome
Paroxysmal nocturnal hemoglobinuria
If used (see later discussion), warfarin is started the evening of initial diagnosis with a loading dose of 2.5 to 10 mg orally; 5 mg is recommended in most patients. Healthy patients younger than 60 years may need a 10-mg loading dose, whereas those older than 85 years, frail and elderly, should start with 2.5 mg. Warfarin is titrated to an international normalized ratio (INR) of 2-3. Use of warfarin affects all the vitamin K–dependent proteins. The first coagulation factor to be reduced by warfarin therapy is factor VII, resulting in prolongation of the INR. However, the full antithrombotic effect of warfarin does not occur until factors X and II levels have decreased. This decrease will take an additional 24 to 48 hours after factor VII levels are reduced. This is the rationale why patients should overlap heparin and warfarin therapy for several days. In patients with acute thrombosis, warfarin should never be started as the sole therapy. Outcomes are superior with the use of heparin before warfarin, and use of warfarin without heparin also puts the patient at risk for warfarin-associated skin necrosis due to interference with protein C and S synthesis.
Duration and Choice of Therapy
Past studies have shown that cancer patients treated with warfarin have 2- to 3-fold higher risk for bleeding and rethrombosis compared with patients who have thrombosis but no cancer. Four studies have shown that cancer patients treated with LMWH have significantly lower rates of recurrent thrombosis but not an increased risk of bleeding and should be considered for treatment for at least 3 months with LMWH. This is especially true for patients with pancreatic, brain, or lung cancer. Presence of cancer is a major risk for recurrent thrombosis, and patients should be treated until they are cancer-free, or lifelong in the presence of metastatic disease. Patients on warfarin who have recurrent thrombosis need to be changed to indefinite LMWH therapy.
Special thrombosis issues in cancer patients
Thrombosis in Patients with Primary Brain Tumors or Metastases
The presence of brain tumors markedly increases the risk of thrombosis. There is often concern about the risk of hemorrhage into the brain if anticoagulation therapy is used. However, this risk is very low for most patients. One study in glioma patients showed identical rates of intracranial hemorrhage in patients on and off anticoagulation therapy. However, patients with brain metastases from choriocarcinoma, melanoma, renal, and thyroid cancer should not be treated with an anticoagulant because of high rates of spontaneous bleeding seen with these tumors. For patients with other tumors, a brain CT should be obtained first to rule out the presence of bleeding before initiating therapy.
Anticoagulation and Thrombocytopenia
Concern regarding the use of anticoagulation therapy in the presence of thrombocytopenia is a valid apprehension; however, scant data exist to guide therapy. A reasonable approach is no anticoagulation at therapeutic dosages when the platelet count is less than 50,000/μL. This is based on indirect data from hemophiliacs, which showed that a platelet count less than 50,000/μL was associated with a marked increase in bleeding. Any form of anticoagulation should be stopped when the count decreases to less than 20,000/μL.
Upper-Extremity Thrombosis
Central venous catheters (CVCs) are essential to many aspects of cancer therapy. The clinically apparent thrombosis incidence for catheters is estimated to be 5% to 30% and can be as high as 40% with peripherally inserted central catheters (PICCs). The signs of catheter thrombosis are nonspecific, and the incidence of thrombosis is thought to be underestimated. CVCs are often coated with sheaths of fibrin soon after introduction. Catheter thrombosis can also be a sign of heparin-induced thrombocytopenia (HIT), because heparin is often used to ensure patency. Unlike lower-extremity thrombosis, the incidence of PE with upper-extremity thrombosis is much lower—only 8% versus 31% in one study.
Therapy starts with removing the catheter, because this will remove the nidus of thrombus. If the patient is not at risk for bleeding, one should consider anticoagulation therapy for 4 to 6 weeks. One can try to “salvage” the catheter by anticoagulating the patients while maintaining the catheter in place, but this approach was associated with a 4% incidence of serious bleeding in a pilot study. Given the low risk of long-term sequelae, there is little indication for thrombolytic therapy.
Prevention of catheter thrombosis is controversial. Most studies have not shown a benefit to prophylaxis against thrombosis with LMWH or warfarin.
Cerebral Vein Thrombosis
Cerebral vein thrombosis occurs commonly in the cerebral sinuses, but in some cases it occurs in the deep cerebral veins. One of the risk factors for thrombosis is the presence of venous hypercoagulable states, including malignancies. Patients with acquired hypercoagulable states, such as paroxysmal nocturnal hemoglobinuria (PNH) or myeloproliferative syndromes, seem to be at increased risk. Another group of patients at risk are those suffering from severe dehydration with sludging of the cerebral blood flow. Finally, patients may have thrombosis due to local irritation of the venous sinuses. The classic presentation of infection-related thrombosis is a cerebral vein thrombosis due to irritation of the transverse sinus by mastoiditis, which is also called “otic hydrocephalus.”
Patients with cerebral vein thrombosis can present with one of two major patterns. The first is with focal neurologic defects due to venous thrombosis, resulting in localized infarction. Infarctions are often hemorrhagic due to continued arterial blood flow, which pumps blood into the infarcted area. Patients with cerebral vein thrombosis will usually present with signs of increased intracranial pressure due to obstruction of venous flow and cerebrospinal fluid reabsorption. Patients will often have severe headaches, nausea, and vomiting and may then progress to coma due to infarction of deep brain structures. Patients may also have reduced vision and blindness due to pressure on the optic nerve. Frequently, patients have a prolonged course lasting for days, with gradual worsening of symptoms.
Especially early in the course of cerebral vein thrombosis, patients may present with nonspecific signs and symptoms. Often patients may be misdiagnosed as having pseudotumor cerebri. This misdiagnosis may occur if only CT scanning is done and found to be normal and the lumbar puncture demonstrates high opening pressures. Diagnosis of cerebral vein thrombosis is best made by MRI and MR angiography, which may best show the venous obstruction.
Cerebral vein thrombosis requires anticoagulation. Despite the frequent presence of hemorrhagic transformation, immediate heparin therapy is associated with an improvement in outcome. In the Einhäupl trial, when patients received a small 3000-unit bolus of heparin, a dramatic improvement in outcome was seen compared with controls. Currently, immediate therapy with either standard heparin or LMWH followed by warfarin is the recommended antithrombotic therapy. Patients with severe neurologic deficits may benefit from angiography and direct thrombolytic therapy of the venous obstruction. Patients with mastoiditis or other local infections should be given anticoagulation therapy for 6 months. Patients with idiopathic thrombosis or cancer-related causes should be given anticoagulation therapy indefinitely.
Adrenal Infarction
The adrenal gland contains a plexus of small veins and venules that receive the secreted hormones of the adrenal gland. This venous structure seems to be prone to thrombosis in several hypercoagulable states. Patients with purpura fulminans may present with adrenal crisis due to thrombosis and the resultant hemorrhagic destruction of the adrenal gland. Patients with HIT may rarely infarct the gland and have subsequent hemorrhage. Finally, patients with the antiphospholipid antibody syndrome (APLS) can have adrenal infarctions. The presentation in APLS patients is often one of adrenal insufficiency that may be overlooked initially due to nonspecific symptoms.
Budd-Chiari Syndrome
Patients with Budd-Chiari syndrome or hepatic vein thrombosis present with the onset of a painful swollen liver and ascites that may progress to liver failure. Several hypercoagulable states are associated with Budd-Chiari syndrome (see Box 2 ), which include the myeloproliferative syndromes—APLS, PNH, and Behçet syndrome. Budd-Chiari syndrome may be the presenting sign of a myeloproliferative syndrome and can occur with normal blood counts.
Therapy is partially dictated by the severity of the liver disease. Because these patients have a hypercoagulable state and are at risk for further life-threatening thrombosis, anticoagulation should be initiated at the time of diagnosis of the thrombosis. Patients who present acutely may be treated with catheter-guided thrombolytic therapy. Patients who present with chronic obstruction may benefit from either surgical or catheter-placed shunts. Patients with hepatic vein thrombosis due to myeloproliferative syndromes do poorly with surgery; therefore, catheter-based shunt approaches should be initially attempted. Despite the presence of hypercoagulable states, shunt thrombosis is uncommon if the patient is given anticoagulation therapy. Patients who undergo liver transplantation and have an identifiable hypercoagulable state should be aggressively anticoagulated to prevent thrombosis of the liver graft.
Portal Vein Thrombosis
With the increase in noninvasive imaging of the abdomen, the incidence of portal vein thrombosis is also increasing. The risk factors for idiopathic portal vein thrombosis are similar to those for Budd-Chiari syndrome. Increasingly, portal vein thrombosis is being recognized as a complication of upper abdominal surgery and laparoscopic colectomy. Patient presentation can range from mild abdominal pain to infraction of the bowel and associated symptoms of an acute abdomen. Data suggest that in provoked portal vein thrombosis, aggressive anticoagulation therapy will aid in the recanalization of the portal vein, and so 3 to 6 months of therapy is reasonable. Patients with idiopathic portal vein thrombosis should be given anticoagulation therapy indefinitely.
Renal Vein Thrombosis
Renal vein thrombosis is most commonly associated with nephrotic syndrome. It is also associated with malignancy but is seen less often with the inherited hypercoagulable states. Patients can present with a clinical spectrum ranging from sudden onset of severe flank pain to a subtle deterioration of renal function. Patients with preexisting renal disease may simply present with worsening renal function. Total venous occlusion will result in hemorrhagic infarction of the entire kidney. Acute thrombosis resulting in renal impairment can be treated with catheter-guided thrombolytic therapy. Patients with more chronic presentations require long-term anticoagulation therapy.
Visceral Vein Thrombosis
DVT and cerebral vein thrombosis are the two most common presentations of hypercoagulable states. Mesenteric veins are the third most common presenting site of thrombosis. Patients usually present with abdominal pain out of proportion to physical findings. Diagnosis can be established at the time of surgery or with CT scanning showing thrombus in the mesenteric vein. Patients may experience extensive bowel infarction with mesenteric vein thrombosis. Once identified, patients with mesenteric vein thrombosis should be treated indefinitely with anticoagulation, even in the absence of an identifiable hypercoagulable state, because this condition has a strong association with recurrent thrombosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




