Heparin-induced thrombocytopenia (HIT) is an immune-mediated adverse drug effect characterized by platelet activation, hypercoagulability, and increased risk of thrombosis, both venous and arterial. A diagnosis of HIT usually signifies that heparin products, including unfractionated and low-molecular-weight heparin, are contraindicated. Although it is uncertain whether heparin continuation really worsens clinical outcomes, it is clear that vitamin K antagonists such as warfarin do worsen outcomes, as they promote microvascular thrombosis, with the potential for limb amputation (venous limb gangrene). Thus, alternative nonheparin anticoagulants are at the forefront of HIT therapy. This review proposes that alternative anticoagulants (danaparoid, fondaparinux) that share certain properties of heparin—namely its irreversible antithrombin-mediated inhibition of factor Xa—and that have relatively long half-lives, have several advantages in the therapy for HIT over short-acting agents that inhibit thrombin directly (recombinant hirudin, argatroban, and bivalirudin).
Heparin-induced thrombocytopenia (HIT) is an adverse drug reaction caused by platelet-activating immunoglobulin G (IgG) antibodies that recognize complexes of platelet factor 4 (PF4) bound to heparin. HIT is highly prothrombotic: at least 50% of affected patients develop thrombosis involving veins, arteries, or even the microcirculation.
Even among patients without clinically evident thrombosis (“isolated HIT”), consensus conference guidelines recommend therapy with a nonheparin anticoagulant, provided that the diagnosis of HIT is confirmed or strongly suspected on clinical grounds; this is because simple discontinuation of heparin, or substitution of heparin with warfarin, is associated with a subsequent risk for symptomatic thrombosis of between 35% and 50%, and for sudden fatal thrombosis of approximately 5%.
A diagnosis of HIT usually signifies—rightly or wrongly—that all heparin preparations, including unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH), are contraindicated. (Whether this is true or not is uncertain; in the author’s experience, severe complications occur at least as often after stopping heparin as when its use is continued.) However, there is little doubt that initiating or maintaining warfarin therapy during HIT-associated hypercoagulability is an important risk factor for microvascular thrombosis, including the syndrome of warfarin-associated venous limb gangrene. Thus, the “contraindication” status of UFH, LMWH, and warfarin during acute HIT necessarily means that novel anticoagulants have attained a prominent role in the management of HIT.
Three nonheparin anticoagulants—recombinant hirudin (r-hirudin), argatroban, and danaparoid—are approved in many jurisdictions for treatment of HIT (an exception: danaparoid is neither approved for HIT nor available in the United States). Two other anticoagulants—fondaparinux and bivalirudin—are approved for non-HIT indications, but are used “off-label” for HIT. These 5 anticoagulants can be divided into 2 groups: (1) long-acting, antithrombin 3 (AT3)-dependent, factor Xa inhibiting oligosaccharides (danaparoid, fondaparinux), and (2) short-acting, AT3-independent (ie, direct), thrombin inhibiting agents (r-hirudin, bivalirudin, argatroban), known as direct thrombin inhibitors (DTIs). The thesis of this review is that for most patients with HIT the indirect factor Xa–inhibiting agents have the greatest therapeutic efficacy .
HIT: a clinical-pathologic syndrome
HIT can be defined as any clinical event (or events) best explained by platelet-activating anti-PF4/heparin antibodies (“HIT antibodies,” or HIT-Abs) in a patient who is receiving, or who has recently received, heparin. Thrombocytopenia is the most common event in HIT, and is observed in at least 90% to 95% of patients, depending on how thrombocytopenia is defined.
HIT is a clinical-pathologic syndrome: thus, the diagnosis requires (1) one or more clinical events (eg, thrombocytopenia, thrombosis, disseminated intravascular coagulation [DIC], necrotizing skin lesions at heparin injection sites, post-intravenous heparin bolus anaphylactoid reaction) that bear a temporal association with a preceding immunizing heparin exposure; and (2) the presence of HIT-Abs ( Box 1 ). Thus, a patient suspected to have HIT but in whom antibodies cannot be detected does not have this diagnosis. Rarely, patients develop a syndrome that mimics HIT on both clinical and serologic grounds but without a preceding exposure to heparin; although named “spontaneous HIT,” affected patients usually have a preceding inflammatory event such as infection or surgery.
Clinical
At least one of:
- •
Thrombocytopenia a
- •
Thrombosis (eg, venous : deep vein thrombosis, pulmonary embolism, venous limb gangrene, adrenal hemorrhage, b cerebral vein thrombosis, splanchnic vein thrombosis; arterial : limb artery thrombosis, stroke, myocardial infarction, mesenteric artery thrombosis, miscellaneous artery; microvascular )
- •
Necrotizing skin lesions at heparin injection sites c
- •
Acute anaphylactoid reactions d
- •
Timing: above event(s) bear(s) temporal relation to a preceding immunizing heparin exposure e
Absence of another more compelling explanation
Pathologic
Heparin-dependent, platelet-activating IgG f
- •
Positive platelet activation assay (eg, serotonin-release assay)
- •
Positive anti-PF4/polyanion-IgG EIA (infers possibility of platelet-activating IgG) g
- •
a >50% platelet count decrease is seen in ∼90% of patients; in 5%–10%, the platelet decrease is 30%–50%.
b Adrenal hemorrhagic necrosis is a consequence of adrenal vein thrombosis.
c Nonnecrotizing lesions (erythematous plaques) are less specific for HIT.
d Usually occur 5–30 minutes after intravenous heparin bolus; rarely, after subcutaneous heparin.
e Typical-onset is 5–10 days after immunizing heparin exposure (usually given intra- or perioperatively); rapid-onset HIT can occur if heparin is given to a patient who already has circulating HIT-Abs, usually due to heparin given in the last 5–100 days.
f Acute serum or plasma should be used for testing, as HIT-Abs are transient.
g In the appropriate context, a strong-positive EIA (IgG-specific or polyspecific assay that detects IgG/A/M antibodies) can be used to infer presence of platelet-activating HIT-Abs.
Laboratory Testing for HIT-Abs
Detectability of HIT-Abs is a key diagnostic criterion. Properly performed, platelet activation assays (using washed platelets) and PF4-dependent enzyme immunoassays (EIAs) are very sensitive for HIT, and thus a negative assay generally rules out the diagnosis. Platelet activation assays (eg, serotonin-release assay [SRA]) detect HIT-Abs based on their characteristic ability to activate platelets at therapeutic (0.1–0.3 U/mL) but not supratherapeutic (10–100 IU/mL) concentrations of UFH. More commonly, commercial PF4-dependent EIAs, which use PF4 bound to heparin or polyvinylsulfonate or platelet lysate proteins, are used to support a diagnosis of HIT. However, approximately 15% to 25% of patients tested yield positive results in an immunoassay and, of these, only one-third to one-half also have a positive test for platelet-activating antibodies ; thus, only approximately 7% to 10% of patients who undergo serologic investigation for HIT truly have this diagnosis. In general, the more abnormal the test result (eg, higher percent serotonin release; higher optical density values in the EIA), the more likely it is the patient has HIT. HIT-Abs are transient, and thus acute serum or plasma should be tested.
Frequency
The frequency of HIT varies widely, ranging from a negligible risk (eg, LMWH given during pregnancy) to a high of 5% to 10% (eg, females receiving UFH prophylaxis for 2 weeks after orthopedic surgery or during therapeutic-dose UFH post-implantation of a ventricular assist device). PF4 and heparin form immunogenic complexes only at certain optimal ratios, and thus stoichiometric considerations influence immunization risk (eg, postoperative patients with higher body mass index are more likely to form anti-PF4/heparin antibodies during fixed-dose prophylaxis with LMWH). Other nondrug risk factors that appear to increase risk of HIT include trauma severity (the greater the degree of trauma, the more likely the patient is to develop anti-PF4/heparin antibodies and clinical HIT ) and the timing of heparin prophylaxis (eg, starting prophylaxis post- vs pre-surgery results in a higher frequency of antibody formation ).
Frequency
The frequency of HIT varies widely, ranging from a negligible risk (eg, LMWH given during pregnancy) to a high of 5% to 10% (eg, females receiving UFH prophylaxis for 2 weeks after orthopedic surgery or during therapeutic-dose UFH post-implantation of a ventricular assist device). PF4 and heparin form immunogenic complexes only at certain optimal ratios, and thus stoichiometric considerations influence immunization risk (eg, postoperative patients with higher body mass index are more likely to form anti-PF4/heparin antibodies during fixed-dose prophylaxis with LMWH). Other nondrug risk factors that appear to increase risk of HIT include trauma severity (the greater the degree of trauma, the more likely the patient is to develop anti-PF4/heparin antibodies and clinical HIT ) and the timing of heparin prophylaxis (eg, starting prophylaxis post- vs pre-surgery results in a higher frequency of antibody formation ).
Pathogenesis
Thrombin Generation
The central concept of HIT is formation of heparin-dependent antibodies of IgG isotype that activate platelets by causing signal transduction through platelet FcγIIa receptors. The target antigen is a complex between (anionic) heparin and (cationic) PF4, a tetrameric member of the CXC chemokine subfamily. HIT-Abs recognize conformationally altered sites on PF4 resulting from its binding to heparin and/or because of close approximation of PF4 tetramers by heparin charge neutralization. LMWH is less likely to trigger both antibodies and HIT, compared with UFH, particularly in females receiving postsurgery thromboprophylaxis. The pentasaccharide anticoagulant, fondaparinux, although similarly immunogenic as LMWH, does not form well the antigens on PF4, suggesting it has an even lower risk of causing HIT.
HIT is a marked hypercoagulability state, as shown by greatly elevated markers of in vivo thrombin generation, for example, thrombin-antithrombin complexes. The explanation for extreme hypercoagulability is uncertain, but could represent pancellular activation, including formation of prothrombotic platelet-derived microparticles, as well as tissue factor expression through activation of monocytes.
The “Timeline” of HIT
The temporal features of antibody formation are consistent with a “point” immunization when heparin is given under circumstances that favor immunization. One such setting is surgery (release of PF4 of activated platelets; perioperative inflammation). HIT-Ab levels first become detectable 4 days (median) after intra- or postoperative heparin administration, followed by the beginning of the HIT-associated platelet count decrease 2 days later (median, day 6) followed by reaching a large-magnitude (>50%) platelet count decrease 2 days later (median, day 8). On average, thrombotic events occur 2 to 3 days later (median, day 10–11), but sometimes occur shortly before the beginning of the platelet count decrease. Antibody levels typically peak between days 10 and 14, and in some patients the platelet count can continue to decrease—or even begin to decrease—after cessation of heparin (“delayed-onset” HIT). Of note, after reaching their peak HIT-Ab levels can decline even with continued heparin administration.
Therapy
The 6 treatment principles of HIT can be summarized as: “2 Do’s ,” “2 Don’ts ,” and “2 Diagnostics ” ( Box 2 ). A crucial tenet is avoiding/postponing warfarin.
| Principle | Comment |
|---|---|
| Two Do’s | |
| Do stop all heparin, including heparin “flushes” and LMWH a | Paradoxically, stopping heparin can worsen risk of thrombosis, because heparin is anticoagulant and HIT-Abs can activate platelets even in its absence |
| Do give an alternative nonheparin anticoagulant (therapeutic-dose) b | Heparin cessation alone is frequently complicated by thrombotic complications |
| Two Don’ts | |
| Don’t give warfarin (give vitamin K if warfarin has already been given) | Warfarin does not block HIT hypercoagulability, increases microthrombosis risk via protein C depletion, and leads to underdosing of APTT-adjusted DTI therapy |
| Don’t give platelet transfusions | Thrombocytopenic bleeding is uncommon in HIT, and platelets might increase risk for thrombosis |
| Two Diagnostics | |
| Test for HIT-Abs | Negative tests rule out HIT; strong-positive tests c are usually seen in “true” HIT |
| Image for lower-limb DVT | DVT is the most common complication of HIT |
Abbreviations: DTI, direct thrombin inhibitor; DVT, deep vein thrombosis; HIT-Abs, HIT antibodies; LMWH, low-molecular-weight heparin; APTT, activated partial thromboplastin time.
a Continuation of heparin (including LMWH) as a therapeutic option is unexplored.
b Danaparoid, fondaparinux, lepirudin, argatroban, bivalirudin (usually in therapeutic doses).
c Anti-PF4/polyanion enzyme immunoassay; serotonin-release assay.
Warfarin is Contraindicated During Acute HIT
Acute HIT is a major risk factor for warfarin (coumarin) necrosis, which can manifest either as venous limb gangrene or classic skin necrosis. The pathogenesis is microthrombosis due to depletion of protein C (vitamin K-dependent natural anticoagulant) in the setting of increased thrombin generation from HIT, that is, disturbed procoagulant-anticoagulant balance.
Venous limb gangrene is characterized by: (1) an underlying hypercoagulability state such as HIT; (2) acral (distal extremity) necrosis in a limb affected by deep vein thrombosis (DVT); and (3) a supratherapeutic international normalized ratio (INR) usually >3.5 (representing a surrogate marker for severe protein C depletion). In contrast, classic warfarin-induced skin necrosis features involvement of skin and subcutaneous tissues at central (nonacral) sites, for example, breast, abdomen, thigh, calf.
The risk of coumarin necrosis in HIT is approximately 5% to 10%, a frequency much greater than the background risk (overall, ∼0.01%). Vitamin K is recommended if a diagnosis of HIT is made after warfarin has already been started, which is aimed to avoid systematic underdosing of DTIs when their therapy is monitored using the activated partial thromboplastin time (APTT), as the APTT is prolonged by warfarin.
A Conceptual Approach to Therapy for HIT
Fig. 1 illustrates key concepts in the management of HIT. The top panel shows the typical “timeline” of HIT-Ab seroconversion. HIT-Ab levels continue to increase after stopping heparin, and typically peak 10 to 14 days after the immunizing heparin exposure. Thus, platelet count declines—and associated hypercoagulability—can intensify despite stopping heparin, and worsen thrombotic events, often manifesting as progressive microvascular thrombosis that complicates preceding macrovascular thrombosis. For example, a patient with HIT-associated DVT can develop venous limb gangrene as a result of progressive microvascular thrombosis, or a patient with HIT-associated arterial thrombosis can develop ischemic limb necrosis—even after embolectomy—because of progressive microvascular thrombosis in the affected limb.
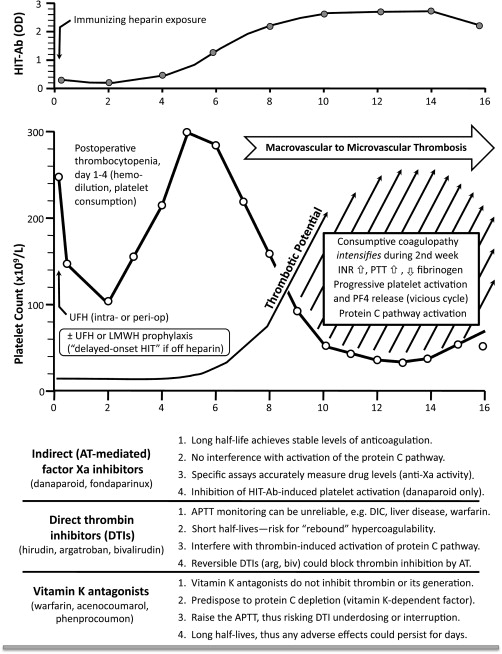
There are several reasons why thrombosis risk is very high during the days 7 to 14 time period: (1) loss of heparin’s anticoagulant effect (including, paradoxically, the iatrogenic factor of stopping heparin because of thrombocytopenia recognition); (2) progressive increase in HIT-Ab levels, often with substantial heparin-independent platelet-activating properties, that is, platelet activation can intensify for several days after stopping heparin; (3) progressive release of PF4 from activated platelets, leading to greater formation of PF4/polyanion complexes on platelet surfaces (self-enhancing vicious cycle), producing greater platelet activation, procoagulant microparticle formation, and increased thrombin generation.
DTIs have potential drawbacks that could limit their effectiveness during this period of intense HIT-associated hypercoagulability. Global coagulation assays (eg, APTT) used to monitor their effects may give misleading information. Also, due to their short half-lives, dose reduction or drug cessation can lead to “rebound” hypercoagulability.
Another issue is potential interference by DTIs of activation of the protein C anticoagulant pathway, which is needed to down-regulate thrombin in HIT-associated hypercoagulability. Warfarin anticoagulation—with protein C depletion—is an important factor explaining microvascular complications in approximately 90% of patients who develop venous limb gangrene in HIT. In theory, DTIs could also promote microvascular thrombosis in certain circumstances, either through inhibiting thrombin-mediated activation of protein C, or (for reversible DTIs, argatroban and bivalirudin) by impeding AT3-mediated inhibition of thrombin. Indeed, cases of venous limb gangrene during DTI-warfarin overlap suggest this period is fraught with risk of progressive microvascular thrombosis.
The bottom panel of Fig. 1 compares the indirect (AT3-mediated) inhibitors of factor Xa with DTIs and vitamin K antagonists. Various factors supporting the advantages of the Xa inhibitors vis-à-vis the other 2 drug classes are summarized.
Nonheparin anticoagulants
Anticoagulants for HIT: the Main Five Agents
Three nonheparin anticoagulants, danaparoid, lepirudin, and argatroban, are approved for treatment of HIT, although approvals vary by jurisdiction. Two other agents, fondaparinux and bivalirudin, are marketed for non-HIT indications, but have a biologic rationale for HIT therapy. These 5 drugs can be classified into 2 groups: those with long-acting AT3-dependent inhibition of factor Xa (danaparoid, fondaparinux) and the AT3-independent DTIs (r-hirudin, argatroban, bivalirudin). Box 3 summarizes key differences between these 2 groups, emphasizing specific implications for HIT. As indicated by the “check marks” (√), the Xa-inhibiting drugs have several advantages over DTIs for management of HIT.
| Indirect AT3-Dependent Factor Xa Inhibitors: Danaparoid, Fondaparinux | Direct AT3-Independent Thrombin Inhibitors: r-Hirudin, Argatroban, Bivalirudin | |
|---|---|---|
| Half-life | √ Long (>16 h): avoids potential for rebound hypercoagulability | Short (<2 h): potential for rebound hypercoagulability |
| Dosing | √ Both prophylactic- and therapeutic-dose regimens a | Prophylactic-dose regimens are not established |
| Monitoring | √ Direct (anti-Xa levels): accurate drug levels obtained | Indirect (APTT): risk for DTI underdosing due to APTT elevation for non-DTI factors |
| Effect on INR | √ No significant effect: thus, simplifies overlap with warfarin | Increases INR: argatroban > bivalirudin > r-hirudin; complicates warfarin overlap |
| Protein C pathway | √ No significant effect | Thrombin inhibition could impair activation of protein C pathway |
| Reversibility of action | √ Irreversible inhibition (AT3 forms covalent bond to Xa) | Irreversible inhibition only with r-hirudin |
| Platelet activation | √ Danaparoid inhibits platelet activation by HIT-Abs | No effect |
| Drug clearance | Predominantly renal | Variable (see text) |
Check mark indicates positive feature in comparison of drug classes.
a Although therapeutic dosing recommended for HIT, availability of prophylactic-dose regimens increases flexibility when managing potential non-HIT situations.
Long-acting AT3-dependent Inhibitors of Factor Xa: Danaparoid and Fondaparinux
Danaparoid
Danaparoid is a mixture of anticoagulant glycosaminoglycans, predominantly low-sulfated heparan sulfate. Danaparoid has both anti-thrombin and anti-Xa activity, although the latter predominates (anti-Xa/anti-thrombin ratio, ∼22). The half-life of its anti-Xa activity is approximately 25 hours. Danaparoid can be given by both subcutaneous and intravenous injection, although the American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines (8th edition) recommends that it be given at least initially by intravenous bolus to ensure rapid attainment of therapeutic concentrations. An intriguing property of danaparoid is that, at therapeutic concentrations, it inhibits HIT-Ab-induced platelet activation in a large percentage of HIT sera. Theoretically, agents such as 2-O, 3-O desulfated heparin could also be used to inhibit platelet activation.
Danaparoid is approved both for HIT and/or non-HIT indications in several jurisdictions, including Canada, Europe, Australia, New Zealand, and Japan; notably, it is not approved for management of HIT in the United States, and has not been available in that market since 2002. Beginning in early 2009, manufacturing problems led to shortages, and the drug continues to have limited availability in some markets.
Danaparoid for treatment of HIT
Danaparoid is the only agent evaluated in a randomized controlled trial for management of HIT. Here, it was superior to the comparator agent, dextran-70. This trial consisted of danaparoid plus warfarin versus dextran-70 plus warfarin. If one assumes that dextran has minimal anticoagulant activity, the comparator essentially consisted of warfarin alone. This design resembles somewhat that of a retrospective study of danaparoid versus comparators that included ancrod and/or coumarin. Pooling these 2 studies ( Table 1 ), danaparoid appears efficacious for HIT. Indeed, danaparoid is the only anticoagulant evaluated for HIT that in comparison with a control group, showed a significantly lower thrombotic rate while at the same time showing a significantly lower major bleeding rate. A retrospective comparison of lepirudin with danaparoid suggested that prophylactic-dose danaparoid is less effective than lepirudin, whereas therapeutic-dose danaparoid showed efficacy broadly comparable to that of lepirudin (see Table 1 ).
| Study | New Thrombosis | Limb Amputation | Composite End Point a | Major Bleeds |
|---|---|---|---|---|
| Chong et al, 2001 | Dan: 3/24 (12.5%) Ctrl: 7/17 (41.2%) RR = 0.30 (0.09, 1.01) P = .063 | Dan: 1/24 (4.2%) Ctrl: 3/17 (17.6%) RR = 0.24 (0.03, 2.08) P = .29 | Dan: 6/24 (25.0%) Ctrl: 10/17 (58.8%) RR = 0.43 (0.19, 0.94) P = .050 | Dan: 0/24 (0%) Ctrl: 0/17 (0%) P = 1.0 |
| Lubenow et al, 2006 | Dan: 11/62 (17.7%) Ctrl: 24/56 (42.9%) RR = 0.41 (0.22, 0.77) b P = .0044 | Dan: 3/62 (4.8%) Ctrl: 4/56 (7.1%) RR = 0.68 (0.16, 2.90) P = .71 | Dan: 15/62 (24.2%) Ctrl: 28/56 (50.0%) RR = 0.48 (0.29, 0.81) P = .0043 | Dan: 8/62 (12.9%) Ctrl: 19/56 (33.9%) RR = 0.38 (0.18, 0.80) b P = .0084 |
| Refs. (pooled c ) | Dan: 14/86 (16.3%) Ctrl: 31/73 (42.5%) RR = 0.38 (0.22, 0.66) b P = .00036 | Dan: 4/86 (4.7%) Ctrl: 7/73 (9.6%) RR = 0.48 (0.15, 1.59) P = .35 | Dan: 21/86 (24.4%) Ctrl: 38/73 (52.1%) RR = 0.47 (0.30, 0.72) P = .0005 | Dan: 8/86 (9.3%) Ctrl: 19/73 (26.0%) RR = 0.36 (0.17, 0.77) b P = .0060 |
| Farner et al, 2001 , d | Dan: 5/53 (9.4%) Lep: 9/114 (7.9%) RR = 1.20 (0.42, 3.39) P = .77 | Dan: 4/53 (7.5%) Lep: 7/114 (6.1%) RR = 1.23 (0.38, 4.02) P = .74 | Dan: 10/53 (18.9%) Lep: 21/114 (18.4%) RR = 1.02 (0.52, 2.02) P = 1.0 | Dan: 2/53 (3.8%) Lep: 7/114 (6.1%) RR = 0.62 (0.13, 2.86) P = .72 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



