Fig. 1
Anatomy of the normal cerebral venous circulation (Reproduced with kind permission from An Atlas of Neonatal Brain Sonography, 2nd edition, by Paul Govaert and Linda de Vries, published by Mac Keith Press (www.mackeith.co.uk) in its Clinics in Developmental Medicine Series 2010 [ISBN 978-1-898683-56-8])
It is helpful to differentiate between two different mechanisms in the pathophysiology of CVT: thrombosis of the cortical veins and thrombosis of the cerebral sinuses. Depending on the extent of the thrombus and the availability of venous collaterals, occlusion of a cortical vein can cause an increase in the venous and capillary pressure and breakdown of the blood-brain-barrier. This process results in localized brain edema, which can progress to venous infarction. A unique characteristic of venous infarcts is that they are often hemorrhagic. Clinically, venous infarcts cause focal neurological deficits and, often, epileptic seizures. The second pathophysiological mechanism, thrombosis of the cerebral sinuses, results in a restricted outflow of cerebrospinal fluid, leading to intracranial hypertension. Major symptoms of intracranial hypertension are headache and decreased visual acuity [10].
5 Risk Factors
In young and middle-aged adults, CVT is three times more common in women than men. This skewed sex ratio is the result of female specific risk factors: oral contraceptive use, hormone replacement therapy, and pregnancy/puerperium [11–14]. These risk factors are generally absent in children and elderly, which explains why in these groups the sex ratio is more evenly distributed [5, 6, 15].
Many different risk factors have been associated with CVT (Table 1). In part these overlap with risk factors for venous thromboembolism (VTE), such as genetic thrombophilia, cancer, obesity, and the previously mentioned female specific risk factors. Other risk factors, mostly those that affect the head and neck region, are specific for CVT. Examples include local infections (otitis, mastoiditis, and meningitis), head trauma, neurosurgical operation, and lumbar puncture. While septic CVT was common in the past, the frequency of infection related CVT has declined over time, probably due to improved antibiotic therapy [10]. Inherited thrombophilias that have been associated with CVT include the prothrombin G20210A mutation, Factor V Leiden mutation, protein S deficiency, protein C deficiency, increased factor VIII levels, JAK2 V617F mutation, and hyperhomocysteinemia [16]. Inflammatory bowel disease and acute lymphoblastic leukemia (ALL) are associated with both VTE and CVT, but, for reasons that are unknown, these conditions are more strongly associated with CVT than VTE [17]. In the case of ALL, the increased risk of CVT appears to be related to the use of asparaginase, possibly in combination with lumbar punctures for intrathecal methotrexate therapy. Anemia has also been associated with CVT and is present in 10–20 % of patients [15, 18]. Severe obesity was recently also found to be a risk factor for CVT. Subjects with a body mass index of 40 or more have an almost ten-fold higher risk of CVT than those with a normal weight. The association between obesity and CVT is especially strong in women of reproductive age who use oral contraceptives [19].
Table 1
Risk factors for cerebral venous thrombosis
Genetic thrombophilia | Cancer |
Especially hematological malignancies | |
Infections | Gender specific risk factors |
Otitis / Mastoiditis | Oral contraceptive use |
Meningitis | Pregnancy / Puerperium |
Systemic infectious disease | Hormone replacement therapy |
Iatrogenic | Medication |
Catheterization jugular vein | Asparaginase |
Neurosurgical intervention | Methotrexate |
Lumbar puncture | |
Miscellaneous | Systemic diseases |
Anemia | Inflammatory bowel disease |
Head trauma | Steroids |
Dehydration | Thyroid disease |
Severe obesity | Behçet disease |
Spontaneous intracranial hypotension | Systemic lupus erythematosus |
Dural arteriovenous fistula | Antiphospholipid syndrome |
Arteriovenous malformation |
At least one risk factor can be identified in 85 % of patients with CVT, and 44 % has multiple risk factors [15]. The most common acquired risk factor for CVT is oral contraceptive use. The reported proportion of women with CVT who use oral contraceptives varies, depending of the country where the study was performed, but percentages of 50 % or higher are not uncommon [2, 15]. Most women with pregnancy related CVT develop symptoms in the puerperium and the risk appears to be increased up to 12 weeks after delivery [12, 20, 21].
6 Symptoms and Signs
The clinical manifestations of CVT are variable, and the disease can be difficult to diagnose. In the International Study on Cerebral Vein and dural sinus Thrombosis (ISCVT), the median delay from symptom onset to admission was 4 days, and the median delay from onset of symptoms to diagnosis was 7 days [15]. A chronic onset of symptoms occurs in less than 10 % of patients and less common in women than men [11].
The most common symptom of CVT is headache, which is found in about 90 % of patients. Although any type of headache can occur, most often it is diffuse and severe. A subgroup of patients present with thunderclap headache, mimicking subarachnoid hemorrhage [22]. Presentation without headache is rare, but occurs more often in older patients, men, and in some associated conditions like isolated cortical vein thrombosis [23, 24]. Focal neurological deficits, such as paresis, aphasia, and hemianopia are found in about half of all patients, and these patients usually have a venous infarct [15]. Seizures occur in approximately 40 % of patients, which is much higher than arterial stroke. A decreased consciousness can also occur, and one in seven patients is comatose at admission, usually as a result of large venous infarcts or bilateral edema of the basal ganglia and thalami [15]. The latter is usually due to thrombosis of the deep venous system and this can also result in behavioral symptoms such as confusion, amnesia, bradyphrenia and mutism [25, 26].
7 Diagnosis
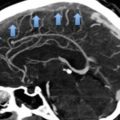
Three different imaging modalities are available to diagnose CVT: CT-venography, MRI with MR-venography, and catheter angiography. Non contrast CT is insufficient to diagnose CVT, but it can show abnormalities that suggest the diagnosis. For example, a thrombus in a dural sinus or cortical vein can appear hyperdense. The latter finding is termed “cord sign” (Fig. 2) [27].
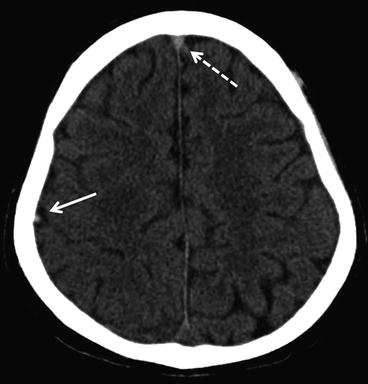

Fig. 2
Axial non-contrast CT-scan of a 27-year-old man with CVT. He had a history of acute lymphoblastic leukemia and presented with generalized seizures. Note the hyperdense signal in the anterior part of the superior sagittal sinus (delta sign; dashed arrow) and in one of the cortical veins overlaying the right hemisphere (cord sign; filled arrow)
The most frequently occluded sinus is the superior sagittal sinus (62 %), followed by the left and right lateral sinus (each around 40 %), and straight sinus (18 %) [15]. Although catheter angiography is still considered the gold standard, it is rarely required anymore. Given the cost and associated risk to the patient, catheter angiography should only be performed if there is doubt about the diagnosis after MRI and CT-venography. When using MRI, it is important to perform both MR-venography and regular MR sequences, because the combination of a signal alteration in the sinus and absent flow on venography is required to make the diagnosis. The precise sensitivity and specificity of MRI and MR-venography are unknown because sufficiently powered studies comparing MRI to catheter angiography have not been performed [27]. Time-of-flight and contrast-enhanced MR-venography can both be used, although the venous system can visualized better with the latter technique [28]. It is also useful to include a susceptibility weighted sequence, since this is the most sensitive technique to visualize a thrombus in a cortical vein [29].
CT-venography is a good alternative to MRI to diagnose CVT. A small study that compared CT-venography to catheter angiography showed a sensitivity of 95 % and specificity of 91 % for the detection of the thrombosis of the cerebral venous system [27]. Advantages of CT-venography include a wide availability, quick image acquisition, and the ability to image patients with a pacemaker or other ferromagnetic devices. Disadvantages of CT venography are the need for intravenous contrast material and exposure to ionizing radiation, which limits its applicability in children and pregnant women. Moreover, CT is inferior to MRI to detect parenchymal lesions and cortical vein thrombosis [24, 30].
Parenchymal brain lesions are found in approximately 40–60 % of patients with CVT, and usually consist of hemorrhagic infarcts or cerebral edema. The shape and size of hemorrhagic lesions can vary from small petechial hemorrhages to large intracerebral hemorrhages (Fig. 3). Juxtacortical hemorrhages, which are small (<2 cm in diameter) hemorrhages that are located just below the cortex, are very typical for CVT and rarely seen in other conditions [31]. Large space-occupying hemorrhagic infarcts of the temporal lobe are usually caused by thrombosis of the vein of Labbé or one of the inferior middle cerebral veins that drain into the transverse sinus [32].
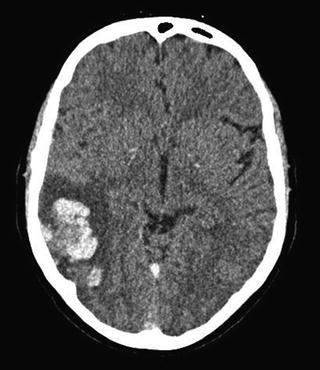

Fig. 3
Axial non-contrast CT-scan of a patient with CVT. In the right hemisphere (left side on the CT) a large hemorrhagic infarct is visible. Darker areas in the lesion indicate edema and the white areas blood
Standard blood tests should be performed in all patients with CVT, consisting of a complete blood count, chemistry panel, prothrombin time and activated partial thromboplastin time [27]. Routine screening for genetic thrombophilia is not recommended, since it usually does not change clinical practice, but it may be performed in selected patients with high a pre-test probability for severe thrombophilia (i.e. recurrent thrombosis, a family history of venous thrombosis, or CVT without a risk factor) [16]. Prior to screening for thrombophilia, it is advisable to consult with a thrombosis specialist. A meta-analysis showed that the sensitivity of D-dimer measurement in patients with CVT is 94 % [33]. Among patients with a chronic headache or isolated headache, however, the sensitivity was considerably lower, 83 % and 82 %, respectively. Since these are exactly the patients in whom D-dimer measurement could be helpful, since there may be no other reason to perform brain imaging other than to exclude CVT, the value of D-dimer measurement in the diagnostic work-up of CVT is limited.
8 Treatment
8.1 Anticoagulation
The primary therapy for patients with CVT is anticoagulation with heparin in the acute phase, followed by oral anticoagulation in the chronic phase. The efficacy and safety of heparin treatment has been investigated in 3 small-randomized trials [34–36]. A meta-analysis showed a pooled relative risk of death and dependency of 0.46 (95 % CI 0.16-1.31) after heparin treatment as compared to placebo [37]. Hence, the limited data that is available suggests a beneficial effect of heparin treatment, but the difference was not statistically significant. Importantly, heparin treatment was not associated with increased risk of hemorrhagic complications. Both low-molecular weight and unfractionated heparin are used to treat CVT, although based on data from two studies, low molecular weight heparin is preferable [38, 39]. The optimal duration of anticoagulant therapy is not known, but most physicians treat for a period of 3–12 months [40]. Factors such as whether the thrombosis was provoked and the preference of the patient should be taken into account when determining the duration of treatment. In some patients, for instance in case of a recurrent thrombosis or in the presence of severe genetic thrombophilia, indefinite treatment with anticoagulation is recommended.
8.2 Endovascular Treatment
During endovascular thrombolysis a catheter is introduced into the cerebral venous system, with the aim to remove the thrombus. Access is usually acquired using a transfemoral or transjugular approach, although direct puncture of a sinus through a burr hole has been reported. Thrombolysis can be achieved using chemical thrombolysis (urokinase or rt-pa), mechanical thrombectomy, or a combination of both. Although many case reports and case series have been published reporting promising results with endovascular treatment, no controlled studies have assessed the efficacy and safety of this therapy [41, 42]. Therefore, endovascular treatment should not be routinely performed in patients with CVT, although it can be considered in selected patients with a severe form of CVT or who deteriorate despite heparin treatment. Currently, there is a randomized controlled trial ongoing in which the efficacy of endovascular therapy is being assessed (TO-ACT trial) [43]. At the time this manuscript was written, 52 patients were included in this study.
8.3 Decompressive Surgery
Decompressive surgery is indicated in a small subset of patients with signs of transtentorial herniation. Clinically, these patients develop a depressed consciousness, with or without 3rd nerve palsy. Imaging will generally shows a large venous infarct with mass effect and shift of midline structures. During decompressive surgery, a hemicraniectomy (or sometimes bilateral) is performed, thereby removing the immediate threat of fatal herniation. Recent studies have shown that this procedure can be lifesaving and result in a good outcome in many patients [44, 45]. The largest retrospective study on this subject included data from 69 patients [45]. The mortality was 16 %, and at follow up 57 % of the patients was functionally independent. Good outcomes have even been reported in patients with advanced stages of transtentorial herniation [46].
8.4 Steroids
The rationale behind the use of steroids in patients with CVT is that it may reduce vasogenic edema.
The efficacy of steroid treatment has only been assessed in one, non-randomised, study [47]. Overall, there was no difference in poor outcome in patients who did or did not receive treatment with steroids, but patients without a parenchymal lesion had a worse outcome if treated with steroids. Although residual confounding may have biased the results of this study, until more data become available, the use of steroids in patients with CVT cannot be recommended, especially not in those without parenchymal lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree