Epidemiology
Venous thromboembolism (VTE) complicates 1 to 2 per 1,000 pregnancies.
1,
2,
3,
4 Furthermore, VTE is the leading cause of maternal death in Western countries, accounting for 1 to 2 deaths per 100,000 pregnancies and 20% to 30% of all maternal deaths.
5,
6,
7 Compared to age-matched nonpregnant women, this implies a 4- to 16-fold risk increase during pregnancy.1 In the postpartum period, the relative risk of VTE has been found to be as high as 60.
8Deep vein thrombosis (DVT) accounts for 85% of pregnancy-related symptomatic VTE, and the majority, that is 65%, occur during pregnancy.
9 Contrary to this, the majority (85%) of episodes of pulmonary embolism (PE) occur in the postpartum period.1 Approximately 22% of episodes of VTE during pregnancy occur in the first, 34% in the second, and 48% in the third trimester.
10 Given the much longer duration of the antepartum period than the postpartum period, the daily absolute risk of VTE is highest postpartum.
Inherited thrombophilias also increase the risk, and absolute risk estimates are summarized in
Table 116.1. The presence of a positive family history for VTE modifies the risk of a first pregnancy-related VTE and in this setting ranges between 1.7% and 14.0%, depending on the type of hereditary thrombophilia.
11,
12 No data are available for the risk of a first pregnancy-related VTE in women with antiphospholipid syndrome. Outside of pregnancy, the persistent presence of lupus anticoagulants including anticardiolipin or
b2-glycoprotein antibodies is associated with a 2- to 10-fold risk increase for a first VTE.
13,
14 If all pregnancy-related VTE is considered, the presence of antiphospholipid syndrome is associated with a 15.8 (95% CI 10.9 to 22.8) times higher risk.
6 It should be noted that in this study, antiphospholipid syndrome was classified by hospital admission code and that this risk increase, at least in part, is likely due to the coexistent history of VTE, which in itself was the strongest risk factor for pregnancy-related VTE (odds ratio [OR] 24.8, 95% CI 17.1 to 36.0).
6 It is unclear what the risk of VTE is in women with antiphospholipid syndrome diagnosed on reproductive clinical criteria such as recurrent miscarriage. In a small study in women with recurrent miscarriage and antiphospholipid syndrome, the risk of subsequent thrombotic events was not increased compared to women with unexplained recurrent miscarriage.
15 The risk of pregnancy-related VTE also increases with age, mode of delivery, and presence of comorbid conditions.
1,
6,
16Despite these strong risk increases, a strong evidence base for the management of pregnancy-related VTE is missing. It is striking that, contrary to nonpregnant patients, diagnostic strategies, therapeutic options, and preventive measures for VTE in pregnant and postpartum women have not been addressed adequately in well-sized observational or intervention studies. Therefore, management is not standardized between physicians, centers, and countries.
Clinical Presentation of VTE in Pregnancy
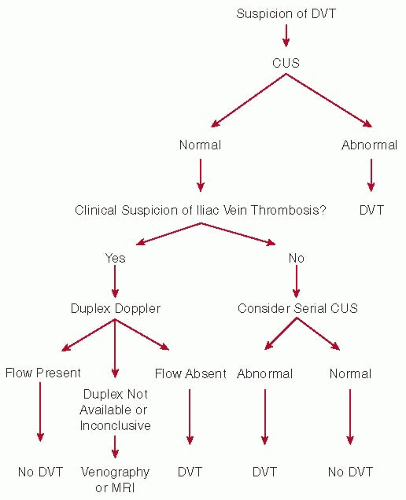
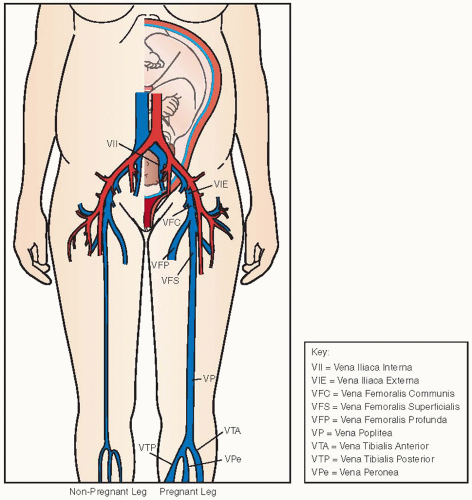
DVT in pregnant patients has a different distribution along the venous system as compared to nonpregnant patients. The predisposition for DVT to occur in the left leg, that is, almost 90% in pregnant patients, is much stronger than in nonpregnant patients (˜60%).
10,
19,
20,
21 Isolated iliac vein thrombosis occurs in 17% of all DVT in pregnant women, whereas this is very uncommon in nonpregnant patients.
20 Furthermore, in contrast to nonpregnant patients, more than 60% of DVT is restricted to the iliac and/or femoral vein, without calf vein involvement.
20
Risks of Anticoagulant Drugs in Pregnant and Postpartum Women
In the next paragraphs, we discuss specific fetal and maternal safety issues for anticoagulant agents. Furthermore, as in the nonpregnant population, the most obvious maternal safety issue of any anticoagulant is the risk of bleeding, both antepartum as well as around delivery and in the postpartum period. These risks are described in the paragraphs of the specific agents if data are available in pregnant women.
Vitamin K Antagonists
Vitamin K antagonists (VKAs) cross the placenta and the induced vitamin K deficiency in the fetus may cause fetal wastage, bleeding, and coumadin embryopathy, a disorder that is characterized by nasal hypoplasia and stippled epiphyses.
37 This teratogenic effect is only present when VKAs are taken in the 6th to 12th weeks of gestation (defined as weeks after the 1st day of the last menstrual cycle). When VKAs are used throughout pregnancy, as is sometimes done in women with mechanical heart valves, the risk of congenital abnormalities is estimated between 3.7% and 6.4%.
37,
38,
39 Although exposure during the second trimester of pregnancy does not lead to overt handicaps, careful examination of school-aged children who had been exposed to VKA in utero showed that 18 out of 274 exposed children (7%) had an increased risk for a “multiple adverse outcome,” that is, growth impairment, minor neurologic dysfunction, IQ < 80, and behavioral problems, in comparison to 2 children out of 231 nonexposed controls (risk difference 5.7%, RR 7.6, 95% CI 1.8 to 32.4).
40,
41 The adverse outcomes were mainly found in boys, and of the affected children, 94% had been exposed during the second and third trimester only. From approximately 36 weeks gestation, the bleeding risk for the fetus during delivery is a reason to avoid VKA during this time.
Women can breast-feed during the use of VKAs; particularly nonlipophilic types such as acenocoumarol and warfarin.
42,
43 Of the lipophilic phenprocoumon, only minute amounts are
excreted in the breast milk that are insufficient to prolong the prothrombin time in infants.
44In a systematic review of VKA use in pregnant women with mechanical heart valves, major bleeding events occurred in 2.5% (95% CI 1.7% to 3.5%) of all pregnancies, most at the time of delivery.
38
Low Molecular Weight Heparin
There is ample experience with low molecular weight heparin (LMWH) in pregnant women. These agents do not cross the placenta and are considered safe to use in pregnancy, based on numerous observational studies.
12,
45 The need for, usually once daily, injections is a nuisance of LMWH, and the long-term use in pregnant women frequently leads to skin reactions, which are mainly type IV delayed hypersensitivity reactions at the injection site.
46,
47,
48 Switching to another LMWH preparation is reported to be indicated in 25% of patients, and of these, about one-third also developed complaints with the use of another LMWH.
46 The incidence of heparin-induced thrombocytopenia (HIT) in pregnant patients who are only treated with LMWH is considered very low (<0.1%),
45 and therefore, the weak, grade 2C recommendation in the most recent American College of Chest Physicians (ACCP) guideline is to not routinely monitor platelet counts. The risk of HIT is assumed to be somewhat higher, between 0.1% and 1%, in women who have been exposed to unfractionated heparin prior to LMWH treatment and monitoring of platelet counts is advised.
49 Small studies of bone density in pregnant women receiving prophylactic doses of LMWH suggest that the use of this medication does not induce more bone loss as compared to pregnant women who did not receive LMWH.
50,
51Although small amounts of LMWH are excreted into breast milk, LMWH is not absorbed after oral intake and this is unlikely to have any relevant effect in breast-fed infants.
12,
52The risk of significant bleeding with the use of LMWH in pregnant women is generally reported to be around 2% and as being mostly related to obstetric causes.
45 These data are based on observational studies including a wide range of doses of LMWH with the majority of prophylactic and intermediate doses. Furthermore, these reports may be biased by selective publication of successful patient histories and underreporting of complications.
53,
54,
55 When therapeutic doses are employed, the incidence of major bleeding was estimated to be 1.7% (95% CI 0.4% to 5.0%) in a systematic review of 15 studies (including 6 case reports) reporting 174 women who had been treated for acute VTE.
45 In a prospective evaluation of 126 pregnant women with acute VTE, the risks for bleeding during pregnancy was 6%, with none of the episodes considered by the authors as major bleeding, although these included episodes of rectal bleeding from endometriosis and haemoptysis.
56 The risk of postpartum hemorrhage (>500 mL of blood loss) was 5% in the first 24 hours after delivery, and secondary major postpartum
hemorrhage occurred in 2% of the women in the days thereafter. In our own study of 83 women who had been treated with therapeutic doses of LMWH, the incidence of postpartum hemorrhage was around 10%, which was not statistically different from a cohort of women who delivered in the same hospital and who were not treated with anticoagulants.
57
Unfractionated Heparin
Unfractionated heparin can be administered subcutaneously and dose adjustments can be made based on activated partial thromboplastin times (APTTs), but the short half-life leads to the need for a twice daily dosing regimen. Furthermore, unfractionated heparin is more frequently associated with osteoporosis, HIT, and type I allergic reactions than LMWH.
12 Long-term unfractionated heparin use is known to cause symptomatic osteoporosis in up to 2% of patients.
58 Thus, unfractionated heparin should be reserved for women with a contraindication to LMWH (mostly chronic renal failure with a creatinine clearance below 30 mL/min) or when LMWH is not available.
12Unfractionated heparin has a high molecular weight and a strong negative charge and does not pass into breast milk.
In a retrospective cohort study of 100 pregnancies in 77 women, the rate of major antepartum bleeding in pregnant women treated with unfractionated heparin was 1% (95% CI 0.2% to 5.4%), which is consistent with reported rates of bleeding associated with heparin therapy in nonpregnant patients.
59
Other Heparin-Like Anticoagulants
Danaparoid and fondaparinux have been and are increasingly being used in pregnancy, although the experience in human pregnancies is limited.
12,
60 Furthermore, minor anticoagulant activity could be detected in fetal cord blood in five neonates of mothers who were treated with fondaparinux, indicating some placental transfer of the pentasaccharide.
61 Thus, in the absence of extensive safety data, danaparoid and fondaparinux should be reserved for women who do not tolerate heparin, most often when all registered heparins lead to type I allergy, skin problems, or HIT.
62Very little is known about the passage of fondaparinux or danaparoid into human breast milk. Since both agents are not absorbed after oral intake, it is unlikely to have any anticoagulant effect in breast-fed infants.
63
Thrombin and Factor Xa Inhibitors
A few case reports have described the use of parenteral thrombin inhibitors in pregnancy, but these data are insufficient to conclude on their safety in pregnant women.
12 There is no place for new oral anticoagulants, since the human reproductive risks of these medications, that is, dabigatran, rivaroxaban, and apixaban, are unknown. The summaries of product characteristics for dabigatran and rivaroxaban describe animal reproductive toxicity.
It is recommended by the manufacturers that breast-feeding women do not use these medications.
12
Thrombolytics
Although thrombolysis is contraindicated in pregnancy, a recent literature review identified 13 case reports that described its use in pregnant patients with massive PE.
64,
65 Recombinant tPA and streptokinase are large molecules that do not cross the placenta, whereas urokinase does.
64 In these severely ill women, fetal death occurred in 15% and preterm delivery in approximately 30% of cases.
The risk of maternal major bleeding complications was 30.8%; 95% CI (9.1 to 61.4).
64