Venous Thromboembolism and Pulmonary Embolism
There are a number of published national or international registries that have collected data on venous thromboembolism (VTE) and other thrombotic events in neonates and children
(Table 121.1).
4,
11,
12,
13,
22 Two neonatal registries from Canada and Germany have prospectively collected data on neonatal thrombosis, while a third from the Netherlands includes data from both neonates and older children.
11,
12,
13 Two registries (from Canada and the United Kingdom) have collected data on older children.
4,
22 Inclusion criteria have varied between registries, particularly with regard to the registration of arterial and central nervous system (CNS) events, and the inclusion or not of asymptomatic events.
The incidence of VTE is dramatically influenced by age in adults such that the estimated incidence of first time deep vein thrombosis (DVT) in those aged 25 to 30 years is around 30 cases per 100,000 persons, which increases exponentially to 300 to 500 cases per 100,000 persons in those aged 70 to 79 years.
14 The incidence of DVT and pulmonary embolism (PE) in children in Canada, using data collected from 15 tertiary care centers, was estimated to be 0.07 per 10,000 children and 5.3 per 10,000 hospital admissions.
22 However, more recently, Raffini et al.
15 reported that the annual incidence of VTE in children discharged from pediatric hospitals in the United States increased from 34 per 10,000 admissions in 2001 to 58 per 10,000 admissions in 2007, representing an increase of 70% in 7 years. The Dutch registry collected data via the Dutch Paediatric Surveillance Unit (DPSU) using a monthly mailing system sent to all pediatricians in primary and secondary care and to specific contact persons in tertiary centres.
13 The overall incidence of venous thrombosis was 0.14 per 10,000 children aged 0 to 18 years and 0.05 per 10,000 children after excluding neonates and nonextremity VTE.
13 The British Paediatric Surveillance unit (BPSU) used a similar methodology,
4 and the estimated incidence of all events was 0.07 per 10,000 children. The first registry of neonatal thrombosis was an international registry involving 64 centers in Canada, the United States, and Europe. Based on data reported from Canada, which was considered to be complete, the incidence of clinically apparent thrombosis was 2.4 per 1,000 admissions to the neonatal intensive care unit (ICU).
12 In contrast, the German neonatal registry reported the incidence of symptomatic events was 0.51 per 10,000 births.
11 The Dutch study reported the incidence of neonatal venous thrombosis as 0.07 per 10,000 children.
13Many other studies have examined the rates of VTE in specific high-risk groups such as children and neonates with central venous access.
23,
24,
25 Children with malignancy, with or without central venous access, are often highlighted as a particular high-risk group, with rates as high as 30% to 50%, although there are many causative factors involved, including insertion technique, site of central line placement type of cancer involved, treatment protocol, and the diagnostic techniques applied.
7,
10,
26,
27,
28 Children with short-term central venous access whilst in pediatric intensive care units have thrombosis in 18.3% to 26% of cases.
29,
30 Children undergoing solid organ transplant have frequent thromboembolic complications,
31,
32,
33,
34,
35 but perhaps the highest risk group in children is in those receiving long-term home parenteral nutrition, with cross-sectional studies reporting thrombosis in up to 66%.
36,
37,
38 With respect to PE, there is limited data on epidemiology. General population retrospective autopsy studies report PE in children at 0.05% to
Intracardiac Thrombosis
Right atrial and intracardiac thrombosis are most commonly diagnosed in children who have central venous access devices extending into the right atrium.
78,
79 Children also may develop intracardiac thrombosis following cardiac surgery, including
after Glenn,
80,
81,
82,
83,
84,
85 Fontan,
86,
87 Norwood procedures,
88,
89 and prosthetic valve replacement.
90,
91,
92,
93,
94,
95,
96,
97,
98,
99,
100,
101,
102,
103,
104,
105,
106These complex surgical procedures are common in children with congenital heart disease, and the underlying surgical procedures may have specific implications for the potential consequences of intracardiac thrombosis.
107 A few specific examples are described below.
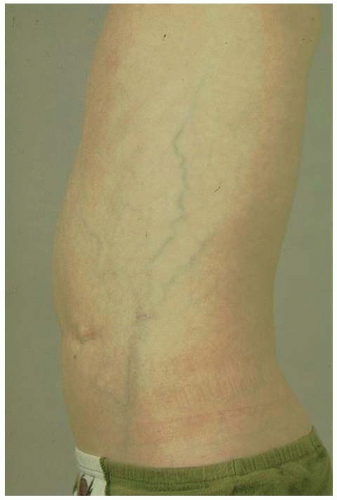
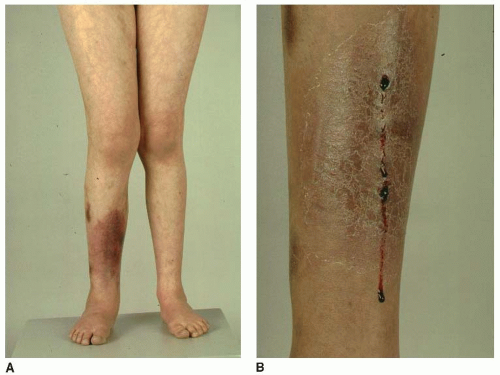
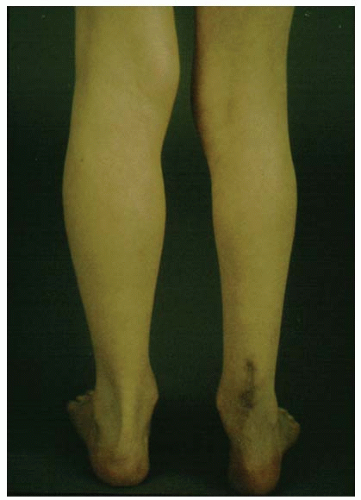
Blalock-Taussig (B-T) shunts are commonly performed in the neonatal period to increase pulmonary blood flow. B-T shunts may be performed as a single palliative procedure with more definitive surgery planned later or as part of a more complex surgical intervention (e.g., Norwood procedure). B-T shunts are performed as either classic or modified B-T shunts. In classic B-T shunts, the subclavian artery is anastomosed directly to the ipsilateral pulmonary artery. The shunt is usually performed on the right side unless there is a right-sided aortic arch, in which case, the shunt is performed on the left. In contrast, modified B-T shunts involve a Gore-Tex tube graft being placed between the subclavian artery and the ipsilateral pulmonary artery. The Gore-Tex tube may be as small as 3 mm in diameter, depending on the size of the infant, but is usually 3.5 or 4 mm in diameter. Blockage of the B-T shunt will compromise pulmonary blood flow and is often a major clinical event requiring immediate surgery. Patients with B-T shunts may have reduced peripheral pulses in the ipsilateral arm and may have measurably reduced growth of the arm. This can be of significance when assessing for postthrombotic syndrome, which may occur in the upper limbs secondary to central venous thrombosis.
The Norwood procedure is the initial surgery performed in infants with hypoplastic left heart syndrome (HLHS). The surgery is performed early in the neonatal period. HLHS usually includes severe hypoplasia of the left ventricle, hypoplasia of the ascending aorta and aortic arch, and critical stenosis of aortic or mitral valves.
The first stage involves the following:
Division of the main pulmonary artery and closure of the distal stump with a patch.
Ligation and division of the patent ductus arteriosus.
Using an aortic or pulmonary artery allograft, the proximal pulmonary artery is anastomosed to the hypoplastic aortic arch, which itself is enlarged to create a large arterial trunk.
The atrial septum is excised to allow adequate interatrial mixing.
A modified B-T shunt (right-sided, Gore-Tex tube, usually 3.5 to 4 mm) from the right innominate to the right pulmonary artery. This will be the only source of pulmonary blood flow.
This procedure results in systemic venous and pulmonary venous return entering into a common atrium. Blood then enters a common ventricle and leaves the heart via a common outflow tract, providing systemic blood flow via the aortic arch and pulmonary blood flow via the B-T shunt.
The implications of this procedure with respect to thrombosis are as follows:
Venous flow has direct access to systemic arterial flow, so deep venous thrombosis, whether central venous line (CVL) related or otherwise, readily causes systemic emboli and potentially arterial ischemic stroke.
A clotted B-T shunt causes loss of entire pulmonary blood flow.
There are substantial intravascular suture lines as potential sources of thrombosis.
The patients will need a subsequent Glenn procedure and eventually completion to a full Fontan procedure. The development of central thrombosis with associated collateral branches can significantly hinder these subsequent surgical interventions. Therefore, increased aggressiveness of any antithrombotic therapy may be appropriate. Even in the absence of symptoms, thrombolysis or surgical thrombectomy may be considered if a central venous thrombosis was thought to be impairing the hemodynamics required for future surgery.
The bidirectional cavopulmonary shunt (BCPS) or bidirectional Glenn procedure is an end (superior vena cava [SVC]) to side (right pulmonary artery) venous shunt. This is a palliative procedure that increases oxygen saturation, but without increasing left ventricular work, and is usually performed in children with univentricular cardiac physiology as the first stage of the two-stage Fontan procedure. The inferior vena cava (IVC) is unchanged after a Glenn procedure.
The implications with respect to thrombosis are that, following a Glenn procedure, the SVC blood flows directly to the lungs without any assistance from the heart. Hence, any reduction in SVC flow, owing to thrombus occlusion, will dramatically reduce pulmonary blood flow. Pulmonary emboli are of specific concern in that if the pulmonary vascular resistance is increased, the patient may become unsuitable for a Fontan completion, which limits long-term survival significantly in the absence of cardiac transplantation. Blood flow from the IVC still bypasses the lungs, meaning that any thrombus in the lower venous system can give rise to paradoxical emboli. Collateral branches around central venous thrombosis may interfere with the ability to complete the Fontan surgery. The threat of being unable to complete the Fontan may be justification for more aggressive antithrombotic therapy (thrombolysis or surgery) than might otherwise be indicated.
There are numerous modifications of the final Fontan procedure, but the basic principles remain the same. Both the SVC (usually done in a first-stage BCPS) and the IVC are anastomosed to the pulmonary arteries. Hence, the pulmonary blood flow is totally passive, depending on venous flow mechanisms without any cardiac pump assistance. The univentricle is then able to function as a left ventricle, providing systemic blood flow. Postoperatively, many patients have deliberate, limited, right to left interatrial shunting via a “fenestration.” The blood flow in such cases increases the risk of paradoxical emboli.
The implications with respect to thrombosis following Fontan surgery are significant. First, the presence of right to left communication continues the potential for paradoxical emboli. Second, any pulmonary emboli that increase pulmonary vascular resistance have an accentuated impact on pulmonary blood flow because the pulmonary pressure cannot be increased to overcome a pressure gradient. The Fontan circuit makes the diagnosis of pulmonary emboli particularly difficult. Depending on the attachment of the SVC and IVC to the pulmonary arteries, isotope may need to be injected into both an arm and a leg to achieve full lung perfusion scanning. As for patients with severe pulmonary hypertension having ventilation-perfusion scans, the transient obstruction of pulmonary capillaries by the macroaggregated isotope-labeled albumin may rarely precipitate a hypoxic episode. Finally, depending on the exact type of Fontan surgery, the risk of late (many years later) thrombosis associated with dysrhythmias may be increased.