The issue of “thromboembolism in intensive care units” deserves dedicated treatment because of some peculiar characteristics of critical patients. In this perspective we synthesize the most relevant: (a) critical patients are a heterogeneous population for thrombotic risk: in patients not receiving thromboprophylaxis was reported a DVT prevalence ranging between 10 and 80 % [10] that was usually observed in patients with major trauma or spinal cord injury, the two subpopulations at the highest risk of thrombosis. In medical-surgical ICU settings a failure rate of prophylaxis as high as 5–15 % was recently shown [11]; (b) in ICU DVT can rarely be diagnosed through clinical data: patients very often cannot communicate symptoms due to their underlying conditions, pharmacotherapy and mechanical ventilation. Signs associated with DVT such as oedema are common in the ICU setting and attributable to many other factors. Diagnosis of DVT needs imaging i.e. ultrasound screening that must be scheduled so that the cost/benefit ratio could be sustainable. (c) The individual risk stratification for DVT in ICU setting is difficult: risk assessment models for DVT diagnosis that are used for out-patients (Wells score) [12], hospitalized medical (Padua score) [13], surgical (Caprini modified score) [14] or trauma patients cannot be applied to critically ill patients. Pretest probability scores developed and validated outside ICU for diagnosis of pulmonary embolism (PE) do not correlated with clinically suspected PE in ICU [15]. In ICU patients not only risk factors such as personal or family history of VET or illness score severity at admission, but also time dependent factors due to newly acquired pathologies, iatrogen interventions or therapies concur to determine thrombotic risk which is dynamic and can change from day to day.; (d) ICU patients can also be at risk of major bleeding [16]. The balance between thrombotic and haemorrhagic risk must be evaluated daily, as it can change suddenly. In medical-surgical ICU patients on thromboprophylaxis the incidence of major bleeding was 6 % and was associated with a twofold increase of ICU and hospital mortality [17]. Dedicated strategies to improve compliance with thromboprophylaxis such as programs of continued education for physicians and nurses and /or electronic order sets and reminders are strongly recommended in ICU [11].
The goal of this review is to offer synthetic and critical knowledge on what we believe to be the main certainties and the main doubts regarding “DVT in ICU setting”.
1 How Many Deep Vein Thrombosis in ICU?
When the “DVT problem in ICU” is investigated, data must be separately analyzed according to (a) the evaluation of DVT clinically suspected or detected by ultrasound surveillance, (b) the use or not of thromboprophylaxis in ICU patients (c) the evaluation of spontaneous and/or CVC-related thrombosis. We remind that catheter-related DVT must be considered those DVT which occur within 72 h from CVC insertion. The difference between DVT prevalence and incidence is also to be taken into account.
2 The Difference Between Prevalence and Incidence of DVT in ICU
Cook et al [3] well describe the difference between prevalence and incidence of DVT in ICU: prevalence takes into account those DVTs that are diagnosed within 48–72 h from ICU admission and that started before admission either during the preceding hospital stay or from trauma or individual thrombotic risk. Data on DVT prevalence are obtainable only when an ultrasound exam for DVT is scheduled within 48–72 h from admission. DVT prevalence depends on factors preceding ICU admission and is independent of ICU quality of care.
DVT incidence in ICU shows how many DVTs are diagnosed 72 h after ICU admission and is the result of the imbalance between prothrombotic stimuli and antithrombotic defence that can occur any time during ICU stay. Indeed, observational studies showed that new-onset DVT can occur at every day independently of the length of ICU stay [4]. No ICU patient can be considered free from risk of DVT (Fig. 2).
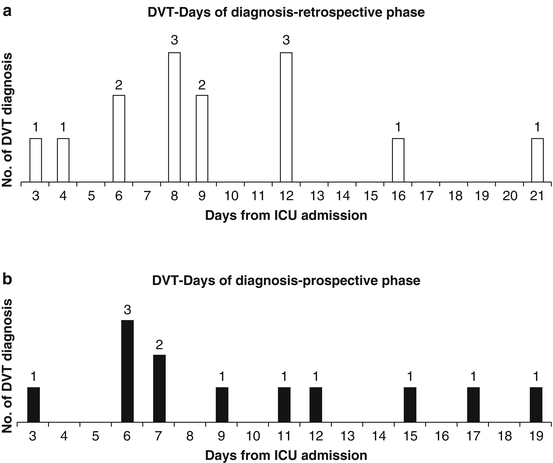

Fig. 2
New-onset DVT can occur at every day independently of the length of ICU stay [4]. During the prospective phase of the study tromboprophylaxis was optimized and DVT incidence decreased from 11 to 4 %, but no difference was observed on the time of occurrence between the prospective and retrospective phase
3 Criticism About Clinical and Ultrasound Diagnosis of DVT in ICU
(a) In the ICU setting, the clinical suspicion of DVT is made difficult by the fact that patients with impaired consciousness through the effect of drugs or simply through their conditions often cannot refer symptoms. Signs that are part of risk assessment models for DVT in hospitalized or out-patients are therefore not easily detectable in ICU patients. Physical examination proved to have no diagnostic utility for DVT in medical-surgical patients [18]. Venous compression ultrasonography (CUS) is the most accurate non invasive test for DVT diagnosis [19, 20], even if CUS has reduced sensitivity in patients with minimal symptoms [21, 22]. Concern about underdiagnosed DVT in the medical-surgical ICU setting is highlighted by studies showing that 10 % [16, 17] to 100 % [23] of DVTs identified by ultrasound screening were clinically unsuspected. Screening test is not recommended by guidelines for DVT prevention [24], but given the common lack of clinical symptoms and signs for DVT in critical patients, DVT diagnosis can only be made by ultrasound screening that allows strict monitoring of the changes in DVT incidence related to the different interventions adopted for prophylaxis improvement. In our opinion a scheduled ultrasound screening program would increase awareness for the need of daily assessment of individual DVT risk burden. Moreover, nowadays in the ICU setting ultrasound is part of the daily clinical assessment of patients and its use for DVT ultrasound screening does not represent a supplementary cost in term of human and tool resources. Finally, we strongly encourage the use of a standard technique for Doppler ultrasound exam through (a) the application of ultrasound compression at six fixed locations from the popliteal trifurcation to the common femoral vein, (b) the measurement of residual thrombus in mm in short axis at each of the CUS fixed locations and (c) scheduled ultrasound test once or twice/a week till discharge (Fig. 3). A standardized methodology would permit optimization of DVT follow-up [3, 4] not only during ICU and hospital stay but also after hospital discharge.
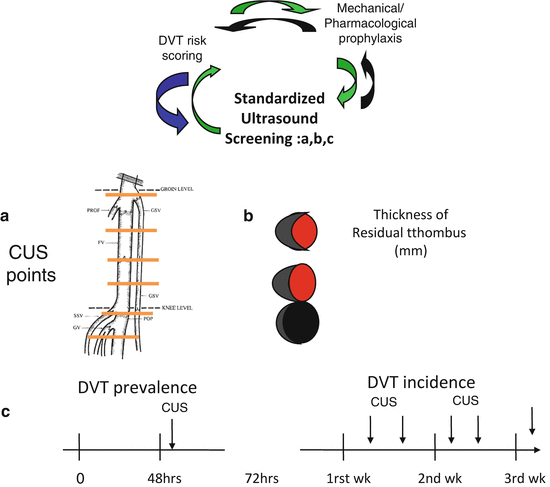

Fig. 3
Standardization of ultrasound exams for the diagnosis of DVT is a key point and is based on (a) the definition of anatomical points on which compressive ultrasound is to be performed, (b) the measurement at of the residual thrombus in mm at each CUS point and (c) the scheduled time of ultrasound exams during ICU stay
It is to be underlined that DVT frequency varies among different studies because of heterogeneity in populations enrolled but most importantly because of the methods of surveillance used and when ultrasound screening is used, because of the frequency of ultrasound test. It is well known that an increased number of ultrasound test in a week, results in a higher number of diagnosed DVT [25]. This means that only studies reporting similar frequency of ultrasound screening are comparable. According to data reported by Cook et al [3] the best risk/benefit ratio is obtained when ultrasound screening is performed twice a week; ultrasound screening carried out less than once a week is therefore unadvisable for a correct control of DVT prophylaxis. When ultrasound screening is scheduled once or twice a week, an incidence of 5–10 % is reported in medical-surgical ICU [3, 4, 26].
4 Does Differentiating Between Symptomatic and Asymptomatic DVT in ICU Have Sence?
Early studies reported that symptomatic or asymptomatic DVT without thromboprophylaxis develops in 13–31 % of medical –surgical critically ill patients [27], whereas in ICU patients receiving LMWH the frequency of DVT at any site ranged between 5.1 and 15.5 % [11]. Due to the extreme difficulty in clinical diagnosis already cited many times, we believe that a reliable distinction between symptomatic and asymptomatic DVT is impossible in ICU settings and that the need for ultrasound screening is absolute when the issue “DVT in ICU” is investigated.
5 What About CVC-Related Thrombosis?
Central venous catheter (CVC)-related DVTs are to be considered those diagnosed within 72 h of CVC insertion. CVCs are included among time dependent acquired DVT risk factors in ICU in all dedicated studies, but some issues must be considered that differentiate CVC-related DVT from idiopathic DVT. The majority of studies on DVT in ICU investigated DVT of the lower limbs, whereas CVC-related thromboses are located in the veins of the upper limbs [28]. The veins of upper limbs are usually not evaluated during ultrasound screening except under specific clinical questions. In patients enrolled in the PROTECT study the incidence of non leg DVT diagnosed in patients with clinical signs or symptoms that prompted ultrasound screening was 2 % [29]. Most importantly, CVC related DVT are mainly due to endothelial damage and in the pathophysiology of CVC-related DVT the role of other risk factors can be different when compared to the role played in idiopathic thrombosis. This is very true when we consider the cannula-related DVT during Extra Corporeal Membrane Oxygenation (ECMO) treatment: the cannulas used in ECMO, expecially the venous cannulas, have a diameter very close to that of the affected vessels with a marked increase in the endothelial damage [30]. Considering all the above points, we believe that CVC-related DVT in ICU deserves dedicated treatment that is beyond the goals of this review.
6 What Is Important to Know About DVT Incidence in ICU?
Data on DVT frequency are extensively reported in literature and according to recent trials DVT incidence in ICU patients under thromboprophylaxis ranges between 5 and 15 %; this means that if in an ICU DVT incidence is above 15 %, an optimization of thromboprophylaxis is called for. Further, it is no longer acceptable for an ICU not to record and not to report the relative data on DVT incidence. A retrospective investigation of what has taken place in the past is necessary in order to form the basis for creating a quality improvement program for the future [4, 26].
6.1 Risk Factors for DVT in ICU
Recognized risk factors for DVT are related to one or more elements of Virchow’s triad (flow stasis, vessel injury and hypercoagulability). In ICU patients, flow stasis plays a major role because of immobility due to trauma, use of sedatives and neuromuscular block that markedly decreases the velocity of limb venous blood flow [31, 32]. In addition, mechanical ventilation and abdominal hypertension, found in many situations, decrease venous return of blood to the heart and can further facilitate the stasis of venous blood in veins of the lower limbs [33] Vessel injuries are mainly due to catheter insertion in central and peripheral veins and/or surgical interventions. Finally, hypercoagulability can be due to sepsis, renal failure or hemodynamic impairment with administration of vasoactive drugs. Under such pathophysiological conditions, the occurrence of additional DVT risk factors during ICU stay added to risk factors present at admission can precipitate thrombosis.
Figure 4 shows that according to the triad of Virchow in ICU setting, several factors occur contemporarily in each patient that facilitate flow stasis and/or endothelial damage and/or hypercoagulabity. We should wonder why not all ICU patients develop DVT. Really, humans are endowed with a very good fibrinolytic system.
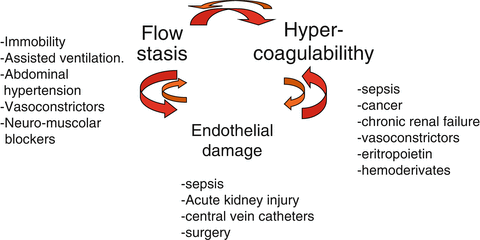

Fig. 4
Virchow’s triad in ICU. Schematic synthesis of different factors that can concur to lower the thrombotic threshold in ICU patients because of their physio-pathological role/s on each of the components of the Virchow’s triad
To calculate the risk for DVT in ICU patients a risk assessment model must be used that takes into account not only the thrombophilic profile of each patient at ICU admission with different items for medical, surgical and trauma patients, but also transient risk factors acquired during ICU stay, the major part related to iatrogenic interventions. In the ICU setting thrombofilic profile is not static, but dynamic; the persistence of DVT occurrence shown in all studies performed in ICU despite an extensive application of thromboprophylaxis, can be partly due to the variability of the thrombotic threshold that is hardly detectable compared with a fixed dosage of anticoagulants. ICU has been called the last frontiere of prophylaxis” [34].
7 DVT Risk Assessment Model in ICU-One for Each ICU?
pre-existent risk factors-a proposal for calculating DVT risk at admission
Pre-existent risk factors are in major part common to the risk factors that in recent years have been included in models for the assessment of thrombotic risk in hospitalized medical and surgical patients. Among those available in literature, the Padua score for medical patients [13] and the Caprini [14] modified score for surgical patients, also applied in the PROF-ETEV study [35] are the most extensively used and we propose that they would be applied in the evaluation of individual thrombotic risk at ICU admission. Remarkably, both Padua and Caprini modified models classify as at high thrombotic risk those patients who have a positive history for TEV, that was also selected together with end-stage renal failure as strongest pre-existent risk factor in ICU medical surgical setting by Cook et al 2005. Moreover, chronic cardiac and/or respiratory failure that select ICU patients at very high DVT risk are identified by Padua and Caprini scores [36]. In ICU intensivists have too short time to do too many things and having an electronic format for thrombotic scoring to complete could be a good methodological approach for the standardized calculation of individual thrombotic profile. In ICU patients the thrombotic risk scoring at admission would not have the goal to decide the start of anticoagulation, but could help to quickly calculate a baseline individual risk threshold which should be daily modified according with the evolution of clinical conditions.
specific of admission diagnosis
Dedicated score are available for hospitalized medical, surgical o trauma patients each taking into account DVT risk factors that play the major role in that specific subpopulation such as underlying inflammatory conditions, minor or major surgery due or not to cancer, number and kind of injured bones. However when patients are admitted to ICU beyond the mentioned risk factors, hemodynamic instability with the need of vasopressor therapy and/or compromised ventilation with the need of mechanical ventilation complicate the clinical picture of the majority of patients. These acute illness are always associated with a very high risk of thrombosis and determine per se the immediate start of anticoagulation [36].
newly acquired and not specific of admission diagnosis
As a whole newly acquired risk factors in ICU can be differentiated in those related to the evolution of the clinical conditions, or secondary to pharmacological or invasive or surgical iatrogenic interventions, as below shown, or rather according to the component of the Virchow’s triad they mainly act on.
- (a)
Related to onset of new medical pathologies
Sepsis, kidney failure, systemic hypotension-hypoperfusion, abdominal hypertension
- (b)
Related to procedures
surgical procedures, peripheral or central catheter insertion
- (c)
Related to mechanical ventilation
- (d)
Related to pharmacological therapies-amine neuromuscular blockers
- (e)
Related to hemoderivate administration.
Risk factors described according to the effect on each component of the Virchow’s triad
Negative action on flow stasis:
Abdominal hypertension, mechanical ventilation, amine, neuromuscular blockers
Negative action on hypercoagulability:
Sepsis, renal failure, amine, hemoderivate, erythropoietin
Negative action on endothelium:
For direct endothelium damage:surgical intervention, CVC
For endothelium dysfunction: sepsis renal failure amine
In recent years a protective effect of statins on DVT was reported and related with their positive effect on endothelial dysfunction; the clinical relevance of these data for thromboprophylaxis in ICU needs dedicated studies [37].
We think that when the pathophysiologic mechanisms of the different DVT risk factors is considered, the dynamic change in individual thrombotic threshold would be better assayed during ICU stay. Dedicated studies are needed to understand whether this approach could have clinical relevance supporting the individual adjustment of pharmacological and/or mechanical prophylaxis.
No “official” risk assessment model is available for ICU patients. Given the high number of risk factors for DVT that may differently coexist and concur to lower the threshold of the risk of thrombosis, risk factors are reported in different studies as having different impact. It is always difficult to apply recommendations by trials or meta-analysis to real clinical practice but it is even more difficult in ICU setting.
We suggest that a specific model should be prepared in each ICU according to the risk factors that characterize that specific population taking into account but not rigidly applying the indications given in the literature.
7.1 DVT Prophylaxis in ICU
- (A)
Pharmacological prophylaxis is recommended in medical-surgical ICU patients
Starting from the first trial by Kapoor et al in 1999 [7] that reported a 50 % risk reduction of screening detected VTE vs placebo, in medical-surgical ICU patients randomly selected and treated with UFU 5,000 units subcutaneously twice daily, in these decades an evidence-based efficacy of pharmacological UFH or LVWH prophylaxis in reducing the risk of VTE in ICU patients has been clearly shown [11, 37]. In a recent systematic review and meta-Analysis of randomized trials on the efficacy and safety of any heparin (UFH or LMWH) thromboprophylaxis vs no anticoagulant prophylaxis, that enrolled 7,226 medical surgical ICU patients, any heparin compared with no heparin was associated with a 50 % lower risk of DVT and of PE with a number needed to prophylax to prevent one DVT of 20, using an assumed control risk of 10 %, and to a number needed to prophylax to prevent one PE of 52, using an assumed control risk of 4 %[38]. Heparin thromboprophylaxis did not influence the risk of major bleeding or mortality.
Laboratory-based variables to define optimal thromboprophylaxis such as thrombin generation and thromboelastometric assay of hypercoagulability have been proposed but dedicated studies are needed to understand how and whether use these data in clinical practice [37].
- (B)
Which heparin?
LWMHs are prepared from UFH by different chemical or enzymatic processes and we know that they have different physical, biochemical and pharmacological properties but we do not know whether this translates in different clinical outcome, specifically in ICU setting. In absence of comparative data among different LWMH, each LWMH should be used at the recommended doses when efficacy and safety data exist [39]. The major advantage of UFH over LMWH is that it avoids a renal clearance that allows UFH administration in patients with impaired renal function. LMWH are associated with a reduced likelihood of heparin-induced thrombocytopenia, that requires administration only once-daily and is commercially available in a unit dose. All the three randomized controlled trials performed between 2000 and 2011 that compared UFH with LMWH for VTE prophylaxis in ICU patients, did not find significant difference in the DVT rate between the two groups [40–42]. Only the multicentric Prophylaxis for Thromboembolism in Critical Care Trial (PROTECT) reported a significant lower incidence of PE (2.3 vs 1.3 %, p = 0.01) in the dalteparin group [43]. Pooled outcomes from the meta-analysis of Alhazzani et al are in agreement with the PROTECT’s results that LMWH was not associated with a lower risk of DVT, but with a reduction of asymptomatic and symptomatic pulmonary embolism when compared with UFH. The risk of DVT, major bleeding, mortality and HIT was similar in the two groups [38].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree