Time
Cumulative mortality risk %
Symptomatic
Asymptomatic
1 year
10
6.2
2 year
18.8
13.1
3 year
27.1
19.8
4 year
36.3
27.9
Fig. 19.1
Frailty has the potential to significantly improve patient selection in the preoperative period for patients undergoing carotid endarterectomy. Applying frailty analysis to patients in the National Surgical Quality Improvement Project, increasing frailty scores correlated with a marked increase risk of 30-day complications in patients undergoing both symptomatic and asymptomatic CEA. Reprinted from J Vasc Surg, 61(3), Melin AA, Schmid KK, Lynch TG, Pipinos II, Kappes S, Longo GM, et al. Preoperative frailty Risk Analysis Index (horizontal axis) to stratify patients undergoing carotid endarterectomy, 683–689, Copyright 2015, with permission from Elsevier
19.5 Abdominal Aortic Aneurysm
Abdominal aortic aneurysm (AAA) is a disease of the elderly most commonly in men who have smoked. AAA is an inflammatory disease of the aorta in which progressive remodeling of arterial wall initiated by smoking with a strong genetic component. The destruction of the aortic wall is caused by the end effector matrix metalloproteinase, which leads to loss of the elastin within the aorta and subsequent dilation of the wall. The presence of an aneurysm is defined by increase in vessel diameter by more than 50 % (usually a diameter over 3.0 cm). Over 95 % of aneurysms are located in the infrarenal location. Once present, aneurysms do not grow longitudinally but rather dilate over time. This growth of the aneurysm occurs in a staccato fashion with potential long periods of absence of growth. An aneurysm once identified warrants careful surveillance. Co-morbidities or physical activity, in spite of a common perception, does not cause rupture. Rather the size of the aneurysm is the most predictive factor for rupture of the aneurysm.
Most aneurysms are identified during the time of radiographic scanning either for unrelated reasons or by clinicians following the current USPSTF recommendations for a one time screen using ultrasound of men ages 65–75 who ever smoked [18]. Physical examination of the aorta is very difficult and especially unreliable in those with an elevated BMI.
Abdominal aortic aneurysms can be assessed utilizing multiple diagnostic imaging techniques. From a screening perspective, ultrasound is able to assess the infrarenal aorta diameter with a sensitivity and specificity nearing 100 %. Because ultrasound has no risk and is reliable it should be performed in patients for both a screening study and those suspected of having an aneurysm. Ultrasound is unable to assess the perivisceral, thoracic and iliac vasculature and therefore once an abdominal aortic aneurysm is identified, a CTA is the most commonly obtained study to fully delineate the concomitant arterial circulation and assess its potential for future repair. The use of magnetic resonance imaging can also document the presence of an aneurysm and help define its morphology. However its use is limited both in routine diagnostic and emergent situations due to the typical delay often experienced in obtaining the study as well as the anxiety many patients experience. Once an aneurysm has been identified, it should be followed at regular intervals if it does not meet size criteria for repair. Currently in the USA, aneurysms are routinely repaired once they reach the size of 5–5.5 cm. This recommendation is based upon multiple trials showing acceptable mortality and morbidity at this aneurysm size. If an aneurysm measures 3–4 cm, it is reasonable to follow this on a yearly or bi-yearly basis with ultrasound. Once the aneurysm reaches 4–4.5 cm, a vascular surgeon will likely reassess the patient at no longer than 1 year intervals, although no definitive recommendations have been established for this surveillance.
Treatment for AAA is specifically aimed at reducing a patient’s risk of rupture and once rupture occurs the mortality with or without surgery is very high. Currently no medical treatment exists for an abdominal aortic aneurysm. Propanolol beta blockade has been shown to be ineffective. Current ongoing trials of antibiotics specifically doxycycline are enrolling patients based on matrix metalloproteinase blockade [19]. Thus for now, surgery remains the only form of treatment that will reduce the mortality risk associated with aneurysm rupture. The risk of the operation must be lower than the risk of aortic rupture. Currently, surgery is indicated when the aneurysm reaches 5–5.5 cm for a standard infrarenal AAA. Mortality from rupture at this size is approximately 1–2 % per year and likely to begin to be greater than the operative mortality. Most importantly intervening on small aneurysms has not been shown to be beneficial. Meta-analysis of the four randomized trials of early AAA repair has not shown a survival benefit and there is a definite burden for the patient with operative repair (Fig. 19.2) [20].

Fig. 19.2
Currently there is no indication for either open or endovascular intervention for patients with aneurysms less than 5 cm based on four well-performed randomized trials demonstrating no benefit to early intervention. From Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015 Feb 8;2:CD001835
Surgical intervention can be open or endovascular. Standard open operations are performed through either a midline laparotomy or retroperitoneal approach with clamping of the aorta and sewing in a bypass graft. This technique has the advantage of eliminating the aneurysm but is associated with hernia formation and potential for postoperative bowel obstructions and aorto-duodenal fistula. More recently endovascular treatment has been popularized with placement of the grafts through the femoral arterial circulation and exclusion of flow into the aortic sac by repairing the aneurysm from within the arterial circulation. This form of treatment typically is associated with a shorter hospital length of stay and recovery time but leaves the aneurysm in situ. Leaving the aneurysm in situ requires close follow-up using CTA with the risk of excessive radiation and inconvenience to the patient resulting from this surveillance. Endovascular repair not uncommonly needs revision because of graft movement and/or re-pressurization of the aortic sac. Nonetheless, with appropriate exclusion of the aneurysm, endovascular repair has been found to have similar mortality rates to open repair over time. In the elderly patient the gain in the immediate perioperative period must be balanced with the burden of repeat imaging to insure a stable aortic repair. Accordingly, the surgeon in discussions with the patient will need to explain carefully the immediate and long-term benefits and burdens of an open or endovascular procedure (Table 19.2).
Table 19.2
The poor long-term survival of octo- and nonagenarians undergoing endarterectomy in long term follow-up
Time | Cumulative mortality risk % | |
|---|---|---|
Octogenarian | Nonagenarian | |
1 year | 10.7 | 16.5 |
2 year | 20 | 28.3 |
3 year | 27.6 | 38.3 |
4 year | 35.6 | 47.3 |
5 year | 43.3 | 56.2 |
19.6 Lower Extremity Arterial Disease
Peripheral arterial disease (PAD) is a disease of the elderly. Approximately 20–25 % of patients over age 75 have disease based on an ankle brachial index of less than 0.90. Approximately 50 % of the population with reduced ABIs will be asymptomatic. Of the remaining patients with reduced ABIs, 40 % will present with intermittent claudication and 10 % will present with critical limb ischemia. Regardless of their presentation, the PAD population is most notable for systemic atherosclerotic disease, which predisposes the patient to a high risk of cardiovascular disease and death. Therefore, any patient with a reduced ABI should be counseled regarding risk reduction activities (smoking cessation and exercise) and treated appropriately for cardiac and cerebrovascular disease, if identified. This impact of the systematic nature of asymptomatic disease is emphasized when examining the survival curve of patients with asymptomatic disease compared to normal patients (Fig. 19.3). Once a patient with PVD becomes symptomatic with either intermittent claudication (IC) or critical limb ischemia (CLI) the survival of the patient worsens [21].
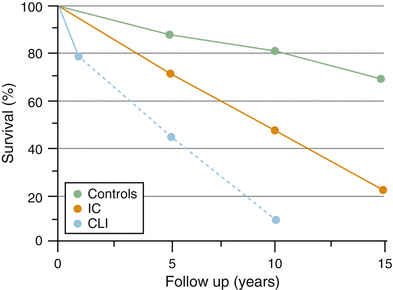

Fig. 19.3




Survival of patients with peripheral arterial disease is markedly decreased in comparison with patients with no evidence of disease. The presence of symptomatic status of a patients PAD markedly worsens long-term survival. Reprinted from Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. Jan;45 Suppl: S5-67, Copyright 2007, with permission from Elsevier
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree