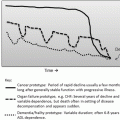
Step 1
Lifestyle modifications
Smaller, more frequent meals
Avoid chocolate, peppermint, acidic foods, or foods that stimulate acid production (caffeine-containing foods)
Stop eating 3–4 h before going to bed
Minimize fats, alcohol, caffeine, and nicotine, especially at night
Sleep with head of bed elevated 6 in
Proton pump inhibitors (re-evaluate after 8–12 weeks )
Esomeprazole (Nexium; 20–40 mg qd)
Lansoprazole (Prevacid; 15–30 mg qd)
Omeprazole (Prilosec; 20–40 mg qd)—available OTC as Prilosec 20 mg
Pantoprazole (Protonix; 40 mg qd)
Rabeprazole (Aciphex; 20 mg qd)
Step 2
Add antacid liquids or tablets for occasional breakthrough
Mylanta, Maalox, Gaviscon, Tums, Rolaids
Add H 2 receptor antagonists (H 2 RAs) at night a
Cimetidine (Tagamet; not recommended in older patients because of drug interactions and delirium risk)
Famotidine (Pepcid; 20 mg qd or bid)
Nizatidine (Axid; 150 mg qd or bid)
Ranitidine (Zantac not recommended in older patients because of increased risk of delirium; 150 mg qd or bid)
Step 3
Surgery
Laparoscopic fundoplication
Nissen fundoplication
Histamine 2 receptor antagonists (H2RAs) are effective for mild symptoms, and avoid the side effects of PPIs such as fracture and Clostridium difficile infection. Cimetidine and ranitidine are not recommended in older patients because of drug interactions and greater anticholinergic effects compared with other H2RAs. While effective, chronic PPI use is associated with an increased relative risk of osteoporosis of 1.97 (>7 years) [3]. There have been reports of other concerns, such as decreased efficacy of clopidogrel against coronary stent occlusion when used in conjunction with PPIs, and increased risk of pneumonia in ventilated ICU patients, and Clostridium difficile infection [4]. Re-evaluate the need for PPIs in patients who have been taking them for longer than 6 months or who had PPIs started for ulcer prophylaxis during hospitalization. Antireflux surgery is reserved for patients with severe refractory GERD with complications. Results from high-volume centers indicate that mortality and morbidity are not increased in patients over 70 years who are at low surgical risk for complications. However, while only 10–15 % of patients have symptoms immediately post-surgery, 5–15 years later 60 % of patients are taking acid suppressive medications.
23.3 Dysphagia
Dysphagia is prevalent in the elderly (20 % compared to 5–9 % in the general population). It is a cause of difficulty eating in 40–60 % of the institutionalized elderly. The incidence of dysphagia increases with increasing obesity [5], as obesity increases the risk of GERD . In a review of patients presenting with dysphagia in a primary care setting, the most common etiologies were GERD (44 %), benign strictures (36 %), esophageal motility disorder (11 %), neoplasm (6 %), infectious esophagitis (2 %), and achalasia (1 %) [6]. Eosinophilic esophagitis (EoE), while rare (18.6 per 100,000 people), can present with difficulty swallowing and food impactions in older patients rather than the atopic symptoms routinely found in the pediatric population [7].
Patients over 65 have multiple changes with aging that predispose to oropharyngeal dysphagia , such as painful or diseased teeth, xerostomia, poorly fitting dentures, slow muscle function resulting in impaired transfer of food into the pharynx, and delayed relaxation of the upper esophageal sphincter (UES) . Barium cinefluroscopic studies of normal adults over age 85 demonstrate that approximately 10 % have silent aspiration of food or fluids. Comorbidities that increase the risk of dysphagia still further include cerebrovascular disease, Parkinson’s disease, multiple sclerosis, Alzheimer’s disease, upper motor neuron diseases, myasthenia gravis, polymyositis, amyloidosis, and a history of surgery or radiation to the oral cavity or neck. In the latter group, recurrence of cancer should be in the differential diagnosis.
Patients with oropharyngeal dysphagia typically cough, gag, choke, or aspirate their food during the initiation of a swallow. Patients may also complain of odynophagia, painful swallowing. Those with esophageal dysphagia often complain of solid foods or liquids “sticking,” “catching,” or “hanging up” in their chest, and may point to their substernal area as the location. This does not always indicate the true location of the problem, as patients with distal esophageal obstruction may have sensations referred higher up in the chest. Dysphagia only to solids often reflects mechanical obstruction, whereas dysphagia to both liquids and solids starting simultaneously suggests a neuromuscular motility disorder. Causes of odynophagia are listed in Table 23.2.
Table 23.2
Causes of odynophagia
1. Medications |
Tetracycline |
Quinidine |
Doxycycline |
Alendronate |
Iron |
NSAIDs |
ASA |
Vitamin C |
Potassium chloride |
2. Infections |
Viral (HSV, CMV, HIV, VZV) |
Bacterial (Mycobacteria) |
Fungal (Candida, Asperigillus) |
3. Acid reflux disease |
4. Malignancy |
Squamous cell carcinoma |
Adenocarcinoma |
5. Miscellaneous |
Ischemia |
Chemotherapy |
Radiation |
Crohn’s disease |
Sarcoid |
Review of a patient’s medication list may suggest pill-induced esophagitis. Elderly patients are at an increased risk for this due to: more medications, decreased saliva production, and anatomical abnormalities compressing the esophagus such as strictures, webs, rings, and vascular anomalies (i.e., enlarged left atrium and dilated aortic arch). History of smoking or heavy alcohol use is associated with increased risk of squamous cell esophageal cancer. Physicians should inquire about these, and look for anemia and unintentional weight loss. Finally, symptoms of GERD should be elicited, as it can cause peptic strictures, Barrett’s esophagus, and adenocarcinoma [2].
A speech-language pathologist can coordinate a cinefluroscopic swallowing study using thin, thick, and solid food materials for patients suspected of having oropharyngeal dysphagia . Patients can be taught proper swallowing techniques and how to modify their posture to improve their swallowing.
In addition to a barium esophagogram, an EGD should be performed to check for malignancy and take biopsies [8]. The diagnostic yield of EGD is around 55 % in the initial evaluation of patients >40 years old who present with heartburn, odynophagia, and weight loss [9]. If upper endoscopy is normal and complaints of dysphagia persist, then esophageal manometry should be performed. Treatment is directed toward the underlying disorder in addition to ensuring adequate nutrition and preventing aspiration. Patients with dysphagia due to decreased esophageal contractility and increased lower esophageal sphincter (LES) pressure (achalasia) may benefit from lower esophageal sphincter (LES) dilation or botulinum toxin injection. In addition to being diagnostic, EGD also offers therapeutic interventions such as dilation, which can be accomplished safely in the elderly (Table 23.3).
Table 23.3
Dysphagia: Conditions for which EGD may provide therapeutic interventions
Benign conditions |
1. Peptic strictures |
2. Schatzki rings |
3. Esophageal web |
4. Eosinophilic Esophagitis |
5. Caustic injury |
6. Radiation injury |
7. Anastomotic stricture |
8. Pill-induced stricture |
9. Cricopharyngeal bar |
Malignant conditions |
1. Esophageal adenocarcinoma |
2. Esophageal squamous cell carcinoma |
3. Pseudoachalasia |
Motility Disorders |
1. Achalasia |
Drugs that decrease smooth muscle contractions (anticholinergics, calcium antagonists, nitrates) may treat diffuse esophageal spasm. Laparoscopic Heller myotomy to open the LES has been performed in older patients with achalasia with reasonable safety and efficacy. If aspiration occurs or the nutritional status of the patient suffers, a feeding jejunostomy or gastrostomy can be considered, but ideally the patient should participate in the decision to proceed with a feeding tube. Current recommendations are to avoid placing G tubes in demented patients, as those have not been shown to improve quality of life. Table 23.4 provides practice tips for dysphagia .
Table 23.4
Practice Tips for Dysphagia in the Elderly Patient
• Dysphagia in the elderly is common and should always be investigated |
• Dysphagia is associated with aspiration, weight loss, and poor quality of life |
• Dysphagia may be oropharyngeal (mostly caused by neurological disorders) or esophageal; the causes of esophageal dysphagia are often indicated by history |
• Common causes of dysphagia include neuromuscular, mechanical, motility, neoplastic and inflammatory conditions |
• Check history of smoking, alcohol use, review medications, do neurologic exam |
• EGD can be diagnostic and therapeutic |
• Patients considered for a feeding tube should be able to participate in the decision |
• Esophageal cancer usually presents in an advanced stage in the elderly, with symptoms of progressive dysphagia and weight loss |
• Surveillance of Barrett’s esophagus should be performed at 1–3 year intervals to detect early adenocarcinoma |
23.4 Peptic Ulcer Disease
Peptic ulcer disease (PUD) refers to both gastric (GUs) and duodenal ulcers (DUs), with the two most common causes being NSAIDs and H. pylori [10]. Approximately 5 million cases of PUD will occur this year in the USA, and the demographics are shifting towards older age of presentation. Older people are more likely to suffer complications of PUD, including hospitalization, need for blood transfusions, emergency surgery, and death. Patients may present with overt bleeding with hematemesis or coffee-ground emesis, or occult bleeding with anemia. Older patients are less likely to have epigastric pain than younger patients, due to decreased visceral sensitivity. About half of patients have minimal pain, and complications such as perforation are more common in this age group [11]. Patients should be asked about a history of PUD ; use of aspirin, NSAIDs, and oral anticoagulants; and previous diagnostic studies (upper GI series, testing for H. pylori). Upper endoscopy should be performed in patients suspected of having PUD to identify the lesion, perform a biopsy for H. pylori, rule out a malignancy, and initiate endoscopic therapy if necessary [12]. Morbidity and mortality of GI bleeding is higher in patients over 70 due to a higher risk of continued hemorrhage causing hypotension and cardiac ischemia. If an ulcer is found, therapy should be initiated with a PPI for at least 8 weeks. NSAIDs and aspirin (including 81 mg ASA) should be stopped [13]. If the patient is found to be H. pylori positive, therapy with antibiotics and a PPI should be started. In the case of a GU, a follow-up EGD should be performed 8–12 weeks later to confirm healing and rule out malignancy. Patients with a prior history of PUD who did not have a significant bleed, and who require chronic NSAID or aspirin use should be treated concurrently with a PPI or misoprostol. Both agents reduce the risk of PUD in chronic NSAID users, although the PPIs are generally better tolerated. Older patients with hemorrhage or perforation should avoid NSAIDs and ASA, as risk of bleeding even with prophylaxis is high and outweighs potential benefit (Table 23.5).
Table 23.5
Practice tips for peptic ulcer disease in the elderly
• Peptic ulcer disease is usually caused by NSAIDs or Helicobacter pylori |
• Complications of peptic ulcer disease are more common in the elderly and morbidity and mortality are higher in this age group |
• PUD in the elderly may present without pain, particularly with NSAID use, and hemorrhage or perforation may be the first sign of an ulcer |
• Dyspepsia is a common complaint in the elderly and requires endoscopy to rule out ulcer or cancer |
• Consider depression as a cause of dyspepsia in an older patient with a negative workup and other symptoms of depression |
• A CT scan of the abdomen may be helpful to diagnose abdominal pain, as elderly patients often present with atypical symptoms of diseases such as cholecystitis, appendicitis, and renal stones |
• Mesenteric ischemia is a diagnosis often missed in older adults: consider it if pain occurs after meals and is progressively worse with time |
23.5 Dyspepsia
Dyspepsia is defined as chronic or recurrent pain or discomfort in the upper abdomen with or without nausea, bloating, early satiety, or reflux and affects 20–30 % of older adults. Dyspeptic pain lasts for hours, distinguishing it from spasmodic pain of colonic contractions or renal stones. There is overlap with the symptoms of cholecystitis and patients often are evaluated for gallbladder disease. It is important to distinguish patients with structural problems such as ulcers from those with “functional” or non-ulcer dyspepsia. Patients should be asked about unintentional weight loss, odynophagia, dysphagia , prior PUD, pancreatitis, biliary tract disease, bleeding, prior trauma, a family history of GI tract cancer, and evidence of blood loss or jaundice. H. pylori infection accounts for a significant number of cases of dyspepsia in patients aged <60. Older patients are more likely to be infected but most are asymptomatic. Non-invasive tests for H. pylori infection that can be done in the outpatient setting include H. pylori serum antibody , urease breath testing, and H. pylori stool antigen .
If prevalence in community is below <20 %, then a H. pylori antibody test (iGG) will have a low positive predictive value, as a positive result is more likely to be a false positive than a true indication of infection. A negative test has a high negative predictive value (>95 %). Both the urease breath test and stool antigen test for active infection can be used before and after treatment. Both have an excellent positive predictive value and negative predictive value of over 90 % regardless of prevalence [14]. If urease breath testing is performed, bismuth and antibiotics need to be stopped for at least 28 days, and PPIs discontinued for at least a week prior to testing due to suppression of active infection by these agents. Stool antigen detection in the setting of use of PPIs or antibiotics may also be affected for the same reason.
In addition to other non-invasive tests for abdominal pathology (complete blood count (CBC), erythrocyte sedimentation rate (ESR), liver function tests (LFTs), electrolytes, amylase, and lipase), consider performing upper endoscopy in H. pylori+ older patients to rule out ulcer and cancer before initiating triple therapy. If H. pylori testing is negative, endoscopy is normal and symptoms persist, then it is reasonable to check for cholecystitis and gastroparesis. In older patients with persistent symptoms, workup should include a CT scan of the abdomen with both oral and intravenous contrast if renal function does not preclude use of IV contrast. If no organic cause is found, patients are categorized as having non-ulcer dyspepsia. There is little data to support routine use of antacids, antimuscarinics, or sucralfate. Routine treatment with H2RAs is of slight benefit, but better results are obtained with once-or twice-daily PPIs in patients with burning pain or pain relieved by food. This suggests that these patients have GERD or some effect of acid on gastroesophageal motility.
Non-ulcer dyspepsia may be the presenting symptom for depression with somatization. Data from the Rome III classification of GI motility disorders supports a relationship between chronic abdominal pain and depression based on evidence that patients with chronic abdominal pain (without irritable-bowel-type relief with defecation) respond better to antidepressants than GI-directed medications [15]. Somatic manifestations of depression (chest pain, abdominal pain, nausea, and early satiety) are more common in the elderly. While there are no controlled studies of selective serotonin reuptake inhibitors in treatment of dyspepsia in older patients, if there other symptoms and signs of depression, a trial of antidepressants may be warranted. Choice should be guided by the side effect profile, as some antidepressants (e.g., tricyclics, mirtazapine) may worsen other common conditions such as constipation .
23.6 Gastric Cancer
In 2002, the number of new cases of gastric cancer reached 900,000; most were in patients older than 60 [16]. Gastric cancer is increasing in the elderly worldwide, while it is decreasing in younger cohorts. The overall 5-year survival rate is estimated at 16 %. Nearly 95 % of gastric cancers are adenocarcinomas, followed by lymphoma at 4 %. Stromal tumors (GISTs), carcinoids, and sarcomas make up 1 %. Risk factors for gastric cancer include chronic atrophic gastritis, H. pylori, pernicious anemia, family history of gastric cancer, partial gastrectomy, tobacco use, alcohol use, and consumption of large quantities of salted or smoked foods containing nitrites and nitrates. Presenting symptoms are often nonspecific (nausea, early satiety, epigastric fullness, intermitted vomiting, weight loss, and abdominal pain). Physical examination may reveal a mass, a succussion splash from gastric outlet obstruction, or peripheral lymphadenopathy. By the time symptoms or physical examination findings are apparent, patients usually have advanced disease. There are no specific chemical tests for gastric cancer, although CEA is often elevated, which can be used to monitor treatment. Gastric cancer is best detected by upper endoscopy. CT scanning with contrast, or MRI can assess depth of tumor invasion and lymphadenopathy. Endoscopic ultrasonography and positron emission tomography scans are increasingly used to improve tumor staging, as patients undergoing EUS are 1.26× more likely to have >15 lymph nodes examined and undergo both pre-and post-operative chemotherapy (Table 23.6) [17]. The general approach to the older patient with cancer is discussed in Chap. 26, Geriatric Oncology.
Table 23.6
Practice tips for gastric cancer
• Symptoms of gastric cancer are nonspecific, and diagnosis is often delayed |
• Gastric cancer is most common in China, Japan, Korean, and Eastern Europe, therefore consider this diagnosis in patients from those areas |
• MALT lymphoma, while uncommon, has a relatively good prognosis and appears to be sequelae of chronic H. pylori infection • Patients need continued endoscopic and EUS surveillance for at least 5 years after surgical resection of gastric cancer |
Mucosal-associated lymphoid tissue (MALT lymphoma) , which is confined to the gastric mucosa, has the best prognosis of all gastric cancers . There appears to be an association between this tumor and infection with H. pylori, and treatment of H. pylori (if present) is first line treatment of low-grade MALT lymphoma. Surgery offers the only cure for non-MALT gastric cancer; however, the overall 5-year survival is poor (20–40 %) and operative mortality high (15–25 %). Patients undergoing surgery should have EGD and EUS surveillance at least yearly for at least 5 years. Endoscopic resection of large masses, laser therapy, and stent placement may provide palliation for patients with obstructive symptoms and inoperable disease. Neo-adjuvant chemotherapy may improve survival by a few months. Palliative chemotherapy may prolong survival and preserve quality of life. Both chemotherapy and radiation are used for treatment of high-grade MALT lymphoma.
23.7 Diarrhea
Patients with diarrhea most often complain of frequent stools (>3/day) or loose stools; however, the term diarrhea is also used to describe fecal incontinence or fecal urgency. Most cases of acute diarrhea (lasting <2 weeks) in the elderly are related to viral or bacterial infections, but medications, medication interactions, or dietary supplements should also be considered. Clostridium difficile colitis is more prevalent in the elderly because of colonization during hospitalizations, antibiotic use, and care in institutional settings. C. difficile colonization in long-term-care facilities is estimated to be at least 50 % in the USA. Lactase deficiency can develop acutely after an episode of diarrhea due to other causes such as viral gastroenteritis. This usually resolves, but may take weeks or months.
Causes of chronic diarrhea, lasting >2 weeks, include: fecal impaction, medications, irritable bowel, microscopic or lymphocytic colitis, inflammatory bowel disease, obstruction from colon cancer, malabsorption , small bowel bacterial overgrowth, thyrotoxicosis, and lymphoma. Patients with neuromuscular disease such as Parkinson’s disease who use anticholinergic medications that decrease GI transit are at risk of small bowel bacterial overgrowth and may present with diarrhea.
Celiac disease is an increasingly recognized cause of diarrhea and bloating in older adults. It is not clear whether this develops de novo in later life or reflects chronic undiagnosed gluten intolerance. Uncommon causes of diarrhea in older patients include Whipple’s disease, jejunal diverticulosis, bowel ischemia, amyloidosis, lymphoma, and scleroderma with bacterial overgrowth. An appropriate history and physical examination, including a rectal examination should be performed. Medication history may reveal the cause. A history of weight loss raises concern for malignancy, inflammatory bowel disease (IBD), microscopic colitis, malabsorption, or thyrotoxicosis. Fluid status with orthostatic blood pressure measurement should be assessed in all elderly patients with diarrhea. Stool cultures should be obtained to exclude infection in patients with acute diarrhea accompanied by fever, abdominal pain, or blood in the stool. Routine stool cultures usually give a specific diagnosis in only 20–30 % of cases [18]. This is likely due to the fact that most infectious diarrheas are due to viruses such as rotavirus and Norwalk agent. For chronic diarrhea, qualitative or quantitative stool fat should be checked for steatorrhea, and a TSH for thyroid disease. C. difficile toxin assay of the stool should be obtained if there is recent antibiotic use. Colonoscopy should be performed in patients with a history of weight loss, bloody diarrhea, and diarrhea lasting >4 weeks. Even if the colonoscopy appears normal, biopsies should be taken for microscopic colitis. X-rays and oral and IV contrast CT scan may demonstrate bowel wall thickening with severe enteritis or colitis; they are also useful if complications such as perforation or abscess are suspected. In patients with possible small bowel bacterial overgrowth due to a variety of risk factors such as motility disorders or structural changes in the GI tract that cause slow GI transit, prior use of antibiotics or immune deficiencies [19], a positive breath hydrogen/methane test confirms fermentation of ingested sugars in the small bowel. Serum antibodies to tissue transglutamidase (tTG) are often positive in celiac disease. Diagnosis is confirmed by villous damage and atrophy in small bowel biopsies.
Treatment of diarrhea focuses on the underlying cause if one is found. In patients without sepsis who are C. difficile negative and have no blood in the stool, loperamide (≤8 tablets/day) can be effective in treating symptoms. Diphenoxylate/atropine (Lomotil®) may cause CNS toxicity, and should be avoided, as should anti-spasmodics such as dicylcomine. Bismuth subsalicylate, which has bactericidal action on common bacterial pathogens, can also be used. C. difficile should be treated with oral metronidazole for mild infections, and oral vancomycin for moderate to severe colitis. Elderly patients have a decreased response to metronidazole compared to younger patients (85 % vs. 95 %), and relapse of C. difficile diarrhea is more common in older patients. Antidiarrheal agents should be avoided in C. difficile colitis due to the risk of toxic megacolon. In microscopic colitis, antidiarrheal agents such as loperamide and bismuth subsalicylate can be tried; however, budesonide is the most effective treatment [20]. If small bowel overgrowth is present, bismuth-containing medications may be helpful in mild cases. For severe cases, treatment with 14–21 days of antibiotics eradicates the offending bacteria. If the cause of slow transit is not addressed or is not treatable, then overgrowth is likely to recur. Elimination of gluten is the treatment for celiac disease and improvement in diarrhea usually occurs within 4 weeks, although healing of the small bowel mucosa can take several months. Medication review is helpful in patients with refractory celiac disease, as medications are an unsuspected source of gluten. For those with irritable bowel syndrome (IBS) , a focus on stress and depression reduction, and referral to a nutritionist to discuss a low Fermentable Oligo-Di-Monosaccharides and Polyols (FODMAP) diet may help (Table 23.7).
Table 23.7
Practice tips for diarrhea in the elderly
• Acute diarrhea is usually self-limited and caused by infections. Chronic diarrhea has many causes, and an extensive workup may be needed. |
– Consider early hospitalization or admission to an observation unit for older patients with diarrhea: increased risk of dehydration, falls, and inability to perform activities of daily living |
• Avoid diphenoxylate/atropine (Lomotil®) due to risk of confusion and ileus from atropine |
• Avoid antidiarrheals until bleeding and C. difficile ruled out |
• Chronic diarrhea—check for: |
– metabolic causes (thyroid disease) |
– microscopic colitis |
– medications |
– malabsorbtion |
– small bowel overgrowth (slow transit) |
– celiac disease |
• IBS (FODMAP diet) |
23.8 Diverticular Disease
Diverticular disease is common in industrialized nations and increases with age; >60 % of those older than 70 and nearly 80 % of those older than 80 have diverticular out-pouchings of the colonic mucosa and submucosa. Diverticuli are most common in the sigmoid colon probably due to increased colonic luminal pressures, with constipation and straining. Approximately 15–20 % of older adults with diverticulosis will have a complication such as diverticular bleeding or diverticulitis.
23.8.1 Diverticular Bleeding
While bleeding from the GI tract can have many origins (Table 23.8), diverticular bleeding is a disease of old age. Forty-five percent of all diverticular bleeding occurs in patients over age 80 [21]. It can present with sudden onset of painless hematochezia. Although most diverticula are on the left side of the colon, 70 % of diverticular bleeding comes from right-sided diverticulae [12]. Eighty percent of diverticular bleeding episodes stop spontaneously, however patients should be hospitalized if bleeding persists, if they are hemodynamically unstable, or if blood loss compromises other organ systems. Older patients are at higher risk for poor outcomes with bleeding, and the threshold for hospitalization should be lower than in younger patients. Evaluation of lower GI bleeding usually involves colonoscopy to exclude sources of bleeding such as arteriovenous malformations (AVMs) , ischemia, IBD, and cancer. Diverticular bleeding is a diagnosis of exclusion in patients with diverticuli. If significant bleeding persists, angiography may show the site. In refractory cases, surgical resection of the bleeding area may be required.
Table 23.8




Causes of GI bleeding in older patients
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree