Chapter 49 Varicella-Zoster Virus Infections
Diseases caused by all human herpesviruses, including varicella-zoster virus (VZV), occur with increased frequency in patients with human immunodeficiency virus (HIV) infection.1,2 Among HIV-seropositive children, primary VZV infection (varicella or chickenpox) is associated with a higher rate of complications than is seen among immunocompetent children. An association between recurrent VZV disease (herpes zoster or shingles) and acquired immunodeficiency syndrome (AIDS) has been noted since the onset of the pandemic. As with herpes simplex virus (HSV) infections and tuberculosis, herpes zoster can occur in HIV-infected individuals with relatively high CD4+ T-lymphocyte counts and may be the initial opportunistic infection.3 In most HIV-seropositive patients, herpes zoster presents as a self-limited cutaneous eruption in a dermatomal distribution. A variety of complications have been described that are associated with increased morbidity and occasional mortality. A unique feature of herpes zoster in patients with HIV infection is a propensity for multiple recurrences.
VZV INFECTIONS
Pathogenesis of VZV Infections
Varicella
There is no convincing evidence that varicella acts as a cofactor to accelerate the progression of HIV disease. Although VZV can transactivate long terminal repeat (LTR) sequences of HIV in vitro, clinical studies have not demonstrated an impact of varicella on CD4+ T-lymphocyte counts.4 This is important because use of a live varicella vaccine in HIV-infected children could not be considered if VZV functioned as a cofactor.
Herpes Zoster
As VZV replicates in the skin during acute varicella, some virions are transported via sensory nerves to the corresponding dorsal root ganglia where latent infection is established. VZV periodically reactivates and undergoes limited gene expression, but replication is suppressed by immunity before any clinical symptoms result. The specific immune responses that limit reactivation of VZV in the sensory ganglia are not well defined. In general, the most important factor that predisposes to the development of herpes zoster is a decline or suppression of VZV-specific cellular immunity, which occurs naturally with aging or can be induced by immunosuppressive illness or therapy. Following reactivation and replication in the ganglion, VZV moves via axonal transport along the sensory nerve to the skin where the virus again replicates in epithelial cells, producing the characteristic dermatomal vesicular rash of herpes zoster. In contrast to the varied lesion stages seen in varicella, most zoster lesions are in similar stages of development.
Investigators initially described herpes zoster as an early sentinel marker of HIV seropositivity.3 Although herpes zoster can occur in patients with any CD4+ lymphocyte count, the incidence is higher among individuals with advanced HIV disease.5–7 In a survey of 175 cases reported from an American cohort, the incidence of herpes zoster was substantially higher in patients with CD4+ lymphocyte counts of less than 100 cells/mm3 (4.1% annually) than in patients with counts of more than 500 cells/mm3 (2.2% annually).8 Unlike most other opportunistic infections, herpes zoster continues to occur at increased frequency despite successful initiation of highly active antiretroviral therapy (HAART).9 Some conflicting data exist, but prednisone therapy probably does not significantly increase the risk of herpes zoster in HIV-infected patients.10
Herpes zoster was originally considered a marker for rapid progression of HIV disease. However, larger studies controlled for age and for CD4+ T-lymphocyte counts have clearly demonstrated that the development of herpes zoster is not significantly associated with the rate of disease progression to AIDS.7,11,12
As has previously been observed with infections caused by cytomegalovirus (CMV) and mycobacteria, immune reconstitution following initiation of HAART may be associated with an increased frequency of VZV reactivation in both adults and children.13–16 Between 4 and 16 weeks after beginning combination antiretroviral therapy (containing a protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitor (NNRTI)), the risk of herpes zoster increases two- to fourfold from baseline. In one study, 24 (8%) of 316 patients beginning combination antiretroviral therapy developed herpes zoster after a mean of 5 weeks.15 During the 6 months following the start of combination antiretroviral therapy, the incidence of herpes zoster exceeds 90 episodes per 1000 person-years.13,15 The percentage of CD8+ lymphocytes at baseline and the magnitude of their increase at 1 month after initiation of drug therapy is strongly associated with an increased risk of herpes zoster.13,15,16 The immunologic mechanisms that account for this observation are not fully understood.17 The clinical presentation and natural history of herpes zoster in the setting of immune reconstitution does not differ from that seen in other HIV-infected patients.13,15
Epidemiology of VZV Infections
Varicella
Until the recent widespread adoption of varicella vaccination, chickenpox epidemics occurred annually in the United States (US) during the late winter and early spring, with numbers of cases peaking in March. During the prevaccine era ∼3.8 million cases of varicella occurred each year in the US, which is approximately equal to the annual birth cohort. About 50–60% of varicella cases occurred in children between the ages of 5 and 9 years, and 90% of cases were in children under 15 years of age. Serologic surveys demonstrated that more than 95% of the US population had been infected by VZV by age 20. Introduction of the varicella vaccine in the US in 1995 has resulted in striking changes in the epidemiology of chickenpox and a dramatic decline in incidence. By monitoring vaccine usage and disease activity at three sentinel sites, the Centers for Disease Control and Prevention (CDC) showed that vaccine coverage among preschool-age children increased from 40% in 1997 to 70% in 1999.18 Between 1995 and 1999 the varicella incidence declined 80% in the surveillance areas, accompanied by an attenuation of disease seasonality. The greatest decline in incidence was seen in children aged 1–4 years.18
Herpes Zoster
The annual incidence of herpes zoster in the US has been estimated at 1.5–3.5 cases per 1000 population.19 An incidence of 2.0 cases per 1000 persons would project to almost 600 000 cases of herpes zoster annually in the US. Increasing age is clearly the most important risk factor for the development of herpes zoster. There is a significant increase in the age-specific incidence of herpes zoster beginning at around age 55; individuals over 75 years of age have a herpes zoster incidence of ∼10 cases per 1000 person-years.19,20 These figures predict that an immunocompetent individual living to be 70 years of age has a 10–20% risk of developing herpes zoster at some point during his or her lifetime. Shingles occurs with equal frequency in men and women, and there is no seasonal association.
The other well-defined risk factor for herpes zoster is altered cell-mediated immunity, as seen in patients with lymphoproliferative malignancies, organ transplant recipients, and AIDS patients. Results from several prospective studies have confirmed the incidence rates for herpes zoster in HIV-infected individuals to be ∼30–50 cases per 1000 person-years.6–8,21,22 In a surveillance study conducted in San Francisco, the incidence of herpes zoster among HIV-seropositive men was 29.4 cases per 1000 person-years, compared with 2.0 cases per 1000 person-years among a control group of HIV-seronegative gay men.23 HIV infection was associated with an increased relative risk (RR) of herpes zoster in all age groups (RR 16.9; 95% confidence interval (CI) 8.7–32.6).23 The cumulative proportion of men developing herpes zoster increased linearly; by 12 years after the diagnosis of HIV infection, 30% of the patients had developed herpes zoster. Among patients with herpes zoster, 22% experienced more than one episode of shingles.23 In a similar prospective study conducted in The Netherlands, the incidence of herpes zoster among HIV-seropositive patients was 51.5 cases per 1000 person-years, with a 41% cumulative incidence over 10 years; in the HIV-seronegative control population, the zoster incidence was 3.31 cases per 1000 person-years, with a 10-year cumulative incidence of 3%.7 Prospective studies in Uganda yielded similar results.22 These observations confirm that the incidence of herpes zoster is ∼15-fold higher among HIV-infected individuals than among age-matched seronegative controls. As a corollary, the possibility of HIV infection should be considered in otherwise healthy patients less than 55 years of age who present with herpes zoster. In African populations where the prevalence of HIV infection is high, more than 90% of patients presenting with a new diagnosis of herpes zoster were found to be HIV infected.24
Clinical Presentation
Varicella
Varicella does not appear to be unusually severe in most HIV-seropositive children.25–27 However, the natural history of varicella in this population is difficult to ascertain from the literature, as most published reports are based on retrospective studies of hospitalized patients or referral populations that likely overestimate the frequency of complications. The clinical presentation of varicella is similar to that seen in immunocompetent children, although some investigators have reported a longer duration of new lesion formation and higher median lesion counts.28 In a prospective, case-controlled study of 30 HIV-infected children with chickenpox, 29 of the cases were scored as mild or moderate in severity, even among the children who received no treatment with acyclovir.4 The only serious complication was one severe case of varicella pneumonia.4 The manifestations of varicella were judged to be less severe in HIV-infected children than in children with acute leukemia.4
A variety of varicella complications in HIV-infected children have been reported, although reliable incidence figures are not available. An inverse correlation between CD4+ T-lymphocyte counts and complication rates has been suggested, but not substantiated in other studies. Cutaneous complications of varicella may include hemorrhagic skin lesions or bacterial superinfections. Visceral dissemination of VZV may manifest by disseminated intravascular coagulopathy, pneumonitis, hepatitis, or encephalitis.29 Deaths attributable to chickenpox in patients with HIV infection are rare and are usually due to pneumonitis.30
Following an episode of varicella, HIV-infected children are at high risk for persistent or recurrent VZV infections.4,25,31 In a few reported cases, the cutaneous lesions of primary varicella failed to heal and remained VZV culture-positive; this was usually associated with a very low CD4+ T-lymphocyte count.29 More often, children develop recurrent cutaneous VZV infections months to years after the primary infection. In a population of 480 HIV-infected children, 117 episodes of VZV infection were identified in 73 patients.29 Of the 73 children, 38 (53%) had recurrent VZV infections; the mean interval from the first to second episode was 17 months. Among 22 children with primary varicella who were followed for 24 months, 10 (45%) had recurrent VZV disease. CD4+ T-lymphocyte counts were no different between children who experienced recurrences and those who did not. Five of the recurrences were classic herpes zoster, and the other five were described as ‘recurrent varicella’ with a widespread cutaneous rash.29 In most cases ‘recurrent varicella’ probably results from VZV reactivation and is actually generalized cutaneous zoster (as has been previously described in other immunocompromised populations), although true reacquisition due to failed immune responses occurs occasionally. Seroconversion following varicella was documented by enzyme-linked immunosorbent assay (ELISA) testing of acute and convalescent sera in six of eight HIV-infected children.32
About 95% of HIV-infected adults have antibody against VZV as a result of childhood varicella, and antibody levels are well preserved even in patients with advanced AIDS. However, when chickenpox does occur in HIV-infected adults, the disease may produce significant morbidity, including VZV pneumonia.33 In a series of five HIV-seropositive adults with varicella, three had uncomplicated courses, one had possible central nervous system (CNS) involvement, and one had possible CNS infection plus hepatitis and thrombocytopenia.34 All five patients improved with acyclovir therapy. Four developed VZV antibody, and one had herpes zoster 2 years after varicella.34 Recurrent varicella-like eruptions, as described above in children, have also been reported in adults.35
Herpes Zoster
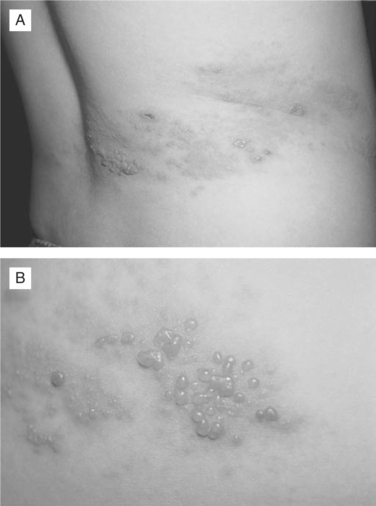
Herpes zoster presents as a painful cutaneous eruption in a dermatomal distribution.36 The inflammatory changes that occur as latent VZV reactivates in the sensory ganglion, produce discomfort in the corresponding dermatome. The patient may report sensations ranging from mild itching or tingling to severe pain that precedes the development of the skin lesions by 1–5 days (or rarely weeks). The cutaneous eruption, appearing in the skin segment innervated by a single sensory ganglion, is unilateral and does not cross the midline (Fig. 49-1A). Overlap of lesions into adjacent dermatomes occurs in 20% of cases. The most common sites for herpes zoster are the thoracic dermatomes (50% of cases), followed by cranial nerve (15%), cervical (15%), lumbar (15%), and sacral (5%) dermatomes. During the acute phase of herpes zoster, most patients experience dermatomal pruritus and pain, which can be quite severe. Patients may also complain of headache, photophobia, and malaise, but significant fever is rare. Skin changes begin with an erythematous maculopapular rash followed by the appearance of clear vesicles (Figs. 49-1A&B). New vesicle formation typically continues for 3–5 days followed by lesion pustulation and scabbing. Bacterial superinfection of the cutaneous lesions occurs in 10–15% of cases.3,6 Skin lesions heal within 2–4 weeks, often leaving skin scarring and permanent pigmentation changes. In rare cases, patients develop dermatomal neuralgic pain but do not progress to the cutaneous eruption phase, a condition termed “zoster sine herpete”. In the normal host, the most frequent complication of herpes zoster is chronic pain, called postherpetic neuralgia (PHN). The incidence and the duration of PHN are markedly increased in elderly individuals.
Patients with deficiencies of cell-mediated immunity, including AIDS, have a high incidence of herpes zoster and an increased likelihood of complications. Most cases of herpes zoster in HIV-seropositive patients are clinically similar to shingles seen in the immunocompetent host, although distinctive features such as frequent recurrences and atypical lesions are well described. Herpes zoster involving multiple nonadjacent dermatomes has occasionally been observed in HIV-infected patients.3,37 A high frequency of herpes zoster involving the first division of the trigeminal nerve (herpes zoster ophthalmicus, or HZO) among HIV-infected patients was reported from both the US38 and Africa.39 However, prospective studies have shown that ∼15% of herpes zoster cases in HIV-seropositive patients involve cranial dermatomes, which is similar to the frequency seen in immunocompetent patients.6,37 Because of the prominent symptoms and cosmetic issues associated with facial herpes zoster, patients with HZO may be more likely to seek care. Studies conducted in Ethiopia and Miami showed that 81 of 85 (95%) and 29 of 112 (26%) patients, respectively, presenting with HZO were found to be HIV seropositive.38,39 HZO warrants aggressive antiviral therapy to prevent ocular complications such as conjunctivitis, keratitis (both acute and chronic), iritis, and uveitis.40
Patients infected with HIV have a much higher frequency of recurrent shingles than is seen in either immunocompetent persons or other populations of immunocompromised patients. About 20–30% of HIV-infected patients develop one or more subsequent episodes of herpes zoster, which may involve the same or different dermatomes.6 The probability of a recurrence of zoster within 1 year of the index episode is ∼12%.41
Whereas, herpes zoster is uncommon in healthy children, shingles is frequently diagnosed in HIV-infected children.4,42 In a prospective study, eight of 30 HIV-infected children with documented varicella subsequently developed herpes zoster.4 The average interval between varicella and zoster was ∼24 months,4,42 although intervals as short as 2 months have been reported. A low CD4+ T-lymphocyte count at the onset of varicella has been reported to correlate strongly with an increased risk of subsequent herpes zoster.4 Investigators at the National Cancer Institute reported a series of 11 HIV-seropositive children with frequently recurring herpes zoster, averaging five episodes per child over 25 months. Of the 58 discrete episodes documented, 29 were characterized as localized herpes zoster, nine cases involved multiple dermatomes, and 20 cases had cutaneous dissemination; there were no cases of visceral dissemination and no deaths.42
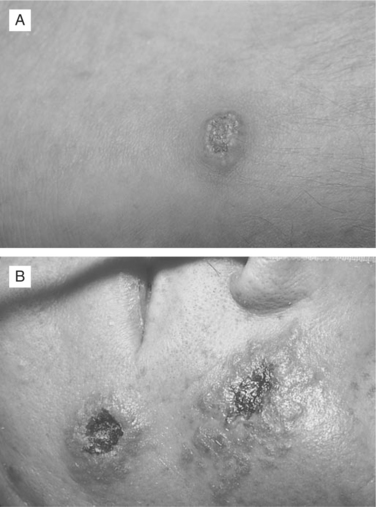
VZV can cause atypical skin lesions in HIV-seropositive patients that are not characteristic of either classic varicella or herpes zoster.43 Aberrant presentations include disseminated varicella-like lesions, disseminated verrucous or hyperkeratotic lesions, disseminated ecthymatous lesions, and disseminated pinpoint papules.44,45 The most common atypical manifestation in HIV-seropositive patients is multiple hyperkeratotic lesions, measuring 3–20 mm in diameter, that follow no dermatomal distribution and may be chronic, persisting for months or years (Fig. 49-2A). A second dermatologic variant is ecthymatous VZV lesions, presenting with multiple large (10–30 mm) punched-out ulcerations with a central black eschar and a peripheral rim of vesicles (Fig. 49-2B). The atypical appearance of these lesions may be linked to abnormal expression of VZV glycoproteins.46 Making the correct diagnosis requires a high index of suspicion, with confirmation provided by viral culture or lesion biopsy. Importantly, a significant number of these atypical verrucous or ecthymatous lesions are caused by acyclovir-resistant strains of VZV. VZV isolates obtained from these atypical lesions should routinely be submitted for antiviral susceptibility testing.
Most herpes zoster-related complications occur in patients with CD4+ lymphocyte counts of less than 200 cells/μL.6,37 A review of 23 cases of VZV dissemination in patients with HIV infection documented that most of these patients experienced zoster involving several contiguous dermatomes, extensive local skin necrosis, and cutaneous dissemination of lesions.47,48 Visceral dissemination of VZV to lung or liver has rarely, if ever, been documented as a complication of herpes zoster in AIDS patients.42,47
The frequency of PHN does not appear to differ markedly between HIV-infected and HIV-seronegative patients with herpes zoster. About 10–15% of HIV-seropositive patients report PHN as a complication following herpes zoster.6 The primary predictors for chronic pain are older age and severity of pain at presentation.49
The primary target organ for herpes zoster dissemination in patients with HIV infection is the CNS.50,51 CNS involvement may occur simultaneously with the cutaneous eruption, follow the acute episode of herpes zoster by weeks or months, or occur in patients with no documented history of cutaneous herpes zoster.52 A variety of neurologic syndromes attributed to VZV infection have been described in HIV-infected patients, including multifocal leukoencephalitis, ventriculitis, myelitis, and myeloradiculitis, optic neuritis, cranial nerve palsies and focal brain stem lesions, and aseptic meningitis.6,53–58 A chronic, progressive form of VZV encephalitis attributed to small vessel vasculopathy has been diagnosed in several AIDS patients.59 Virtually all of these diagnoses of VZV neurologic diseases were made in AIDS patients with markedly depleted CD4+ lymphocytes. Approximately 30–40% of these patients had no recognized recent history of cutaneous VZV infection. In cases in which antecedent herpes zoster had been diagnosed, the skin lesions often preceded the neurologic symptoms by months.
Acute retinal necrosis (ARN) caused by VZV has previously been described in immunocompetent patients. More aggressive variants of this disease have been recognized in patients with AIDS and have been termed varicella-zoster virus retinitis (VZVR), progressive outer retinal necrosis (PORN), or rapidly progressive herpetic retinal necrosis (RPHRN).60,61 The RPHRN syndrome is seen almost exclusively in AIDS patients with CD4+ lymphocyte counts of less than 100 cells/μL.60,62–64 This form of VZV retinitis may occur concurrently with active herpes zoster or, more frequently, develops weeks or months after the acute episode of herpes zoster has resolved. RPHRN can occur after HZO or after herpes zoster involving a remote dermatome. The retinitis begins with multifocal necrotizing lesions involving the peripheral retina. Most patients present with unilateral involvement, but progression to bilateral disease occurs frequently.60,61 The funduscopic examination reveals granular, yellowish, nonhemorrhagic lesions that rapidly extend and coalesce, often resulting in retinal detachment. There is a relative lack of intraocular inflammatory changes. RPHRN rapidly progresses to confluent full-thickness retinal necrosis (which differs from the slow progression seen with CMV retinitis) and results in blindness in 75–85% of involved eyes.60,62 The etiologic role of VZV in most cases of RPHRN has been established by demonstrating the virus by culture or the polymerase chain reaction (PCR) from choroid, vitreous fluid, and retinal biopsies65,66 HSV occasionally causes an identical syndrome.60,67
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree