Introduction
Valvular heart disease (VHD) is an increasingly common and important problem despite the decline of rheumatic fever. One of the principal reasons is the relative and absolute ongoing increase in the elderly population, such that the increased requirements for care of degenerative valve disease now outweigh the decreasing burden of rheumatic heart disease.
The widespread availability of echocardiography has revolutionized investigation by providing accurate and non-invasive assessment of severity and a means of monitoring disease progression, while also allowing progress in the application of interventional techniques and reconstructive valve surgery. The indications for valve replacement surgery have been broadened by improvements in prosthetic valve design and reduced perioperative mortality while the advent of transcatheter aortic valve implantation (TAVI) and percutaneous mitral valve repair offer a less invasive alternative for elderly patients whose frailty and comorbidity may preclude conventional surgery.
Epidemiology and Pathophysiology
VHD was historically caused by rheumatic fever and this remains a major burden in developing countries. However, industrialized countries face a different type of problem: as rheumatic disease has fallen substantially due to improved living conditions and the introduction of penicillin for streptococcal pharyngitis in the 1940s, degenerative VHD is increasingly common.
With an ever-increasing elderly population over the past half century and the increasing availability of diagnostic tools such as echocardiography, the requirements for care of degenerative valve disease now outweigh the decreasing burden of rheumatic heart disease.
Prevalence of Valvular Heart Disease in an Ageing Population
The prevalence data for VHD in the elderly are derived mainly from European and North American studies. In 2006, Nkomo et al.1 collated the results of several large population-based studies (total >28 000 adults) to assess the overall prevalence of VHD and its effect on overall survival in the general population of the USA. The prevalence of VHD increased with age, rising from 0.7% in those aged 18–44 years to 13% in those over 75 years of age (Figure 116.1). The most common valve lesion was mitral regurgitation, followed closely by aortic stenosis, whilst mitral stenosis was very uncommon in this population. There was equal gender preponderance. The presence of VHD also had a statistically significant impact on survival, emphasizing its significance as a healthcare issue.
Figure 116.1 The rising prevalence of valvular heart disease according to age. Frequency in (a) population-based studies and (a) the Olmsted County community.1
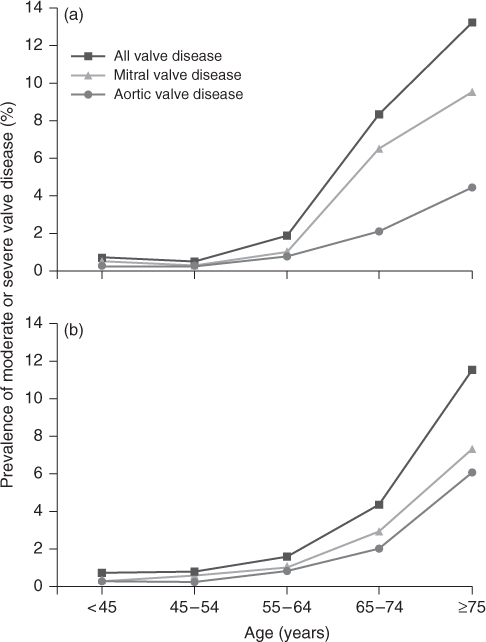
There is also a demonstrable association between the prevalence of VHD and increasing age. In the developed world, low birth rates and increasing life expectancy have inverted the age pyramid. All adult age groups below 65 years will begin to diminish in size throughout most of Europe beyond 2030. In contrast, those aged over 65 years are expected to grow to 107 million by 2025 and 133 million by 2050, with a 180% (19 million to 51 million) increase in those over 80 years of age between 2005 and 2050. Based on a prevalence of VHD of 13% in the over-75 age group, this will result in over 6.5 million new cases of moderate to severe VHD in Europe by 2050.
In the UK, approximately 1 million individuals over 65 years of age are thought to be affected by VHD. This will inevitably increase rapidly as the UK population aged >75 years is projected to rise by ∼50% by 2025. In turn, the impact on healthcare resources is likely to grow substantially.
Aetiology of Valvular Heart Disease
The aetiology and management of VHD were assessed across Europe in 2001 in the European Society of Cardiology Euro Heart Survey on Valvular Heart Disease. Prospective data were collected on 5000 patients with significant primary VHD (according to specific criteria) or infective endocarditis, in both in- and outpatient settings across cardiology and cardiothoracic departments in Europe.
In this hospital setting, the most common native left-sided valve lesion was aortic stenosis (43%), followed by mitral regurgitation (32%), aortic regurgitation (13%) and mitral stenosis (12%). The dominant aetiology of aortic stenosis, aortic regurgitation and mitral regurgitation was degenerative, while mitral stenosis resulted from rheumatic disease in 85%. Valvular regurgitation was caused by a wider range of aetiologies than stenosis, with endocarditis and congenital abnormalities featuring prominently in aortic regurgitation and ischaemia an important cause of mitral regurgitation.
Aortic Stenosis
Detection of aortic stenosis in the elderly has increased with improved diagnostic tools. Echocardiography has revealed mild calcification of the aortic valve in up to 40% of those aged 60 years or over and 75% of those aged over 85 years. The overall prevalence of clinically significant aortic stenosis in those older than 70 years has become more frequent and is ∼1–3%. Recent evidence suggests variation in the left ventricular response to aortic stenosis according to gender: whereas elderly males tend to undergo ventricular dilatation with systolic impairment, females have small, hypertrophied ventricles with preserved contractile function.
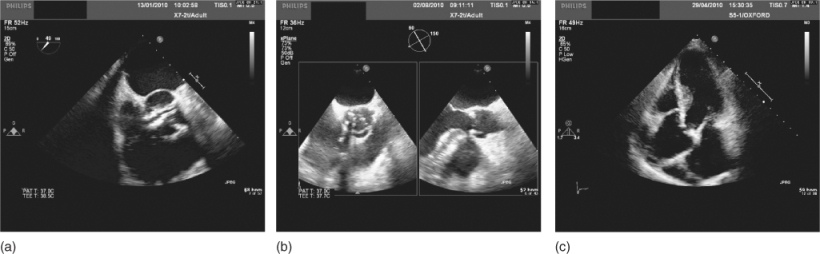
Overall, the most common cause of aortic stenosis is a congenitally bicuspid aortic valve, accounting for over 40% of UK cases (Figure 116.2a). Rheumatic aortic stenosis is now much rarer. Bicuspid valves occur in 0.5–2.5% of the general population and are more common in males. Significant stenosis, caused by progressive calcification (Figure 116.2b and 116.2c), develops in up to one-third of affected individuals, often before the age of 70 years.
Figure 116.2 (a) Transoesophageal echocardiogram (TOE) demonstrating severe aortic stenosis in a bicuspid valve with left ventricular dilatation. (b, c) TOE demonstrating a severely calcified tricuspid aortic valve.
Aortic sclerosis (thickening of the aortic valve without obstruction) is present in 25% of patients over 65 years of age and is associated with age, hypertension, smoking, male gender, low-density lipoprotein levels and diabetes mellitus. Nearly 9% of these patients progress to aortic stenosis over a 5 year period.
Senile degeneration of an anatomically normal aortic valve is increasingly important in the ageing population. The degeneration is related to repeated minor trauma to the valve cusps leading to fibrosis and deposition of calcium, with consequent immobility and stenosis. These changes are unusual before the age of 70 years but the incidence increases rapidly thereafter.
Aortic stenosis is the result of impaired mobility of the valve cusps, leading to reduced systolic excursion and open valve area (normally 3–4 cm2). The consequence is an increase in left ventricular systolic pressure and a pressure gradient across the valve between the ventricle and aorta. The ventricle subsequently attempts to accommodate this pressure increase through hypertrophy that in turn leads to falling compliance of the left ventricle. Symptoms of exertional breathlessness, angina and syncope eventually result. Most patients develop these symptoms before a reduction in ejection fraction, but some present with poor ventricular function and overt heart failure.
Aortic Regurgitation
Aortic regurgitation is less common than aortic stenosis with a prevalence of <1% in the elderly population. The usual aetiology is aortic root dilatation, which appears to occur as part of normal ageing and is more frequent in hypertensive patients. Of the valvular causes, bileaflet valves comprise ∼60% of cases, infective endocarditis 20% and rheumatic heart disease <10%. Rarer causes include inflammatory aortitis (associated with rheumatoid arthritis, ankylosing spondylitis, giant-cell arteritis and syphilis), aortic root aneurysm and ‘silent’ chronic dissection. Acute aortic regurgitation is less common and is usually due to infective endocarditis, but may occasionally arise as a result of aortic dissection, dehiscence of a valve prosthesis or trauma.
Aortic regurgitation is caused by inability of the valve cusps to coapt during the diastolic phase of the cardiac cycle. This may be due to an abnormality of the aortic root or a problem with the valve itself that disrupts valve closure. As a result, a proportion of left ventricular stroke volume (>50% in severe cases) leaks back through the aortic valve in diastole, inducing a compensatory response of ventricular hypertrophy to maintain efficient ventricular function. This compensatory response eventually becomes inadequate, resulting in progressive ventricular dilatation and the onset of exertional breathlessness.
Mitral Stenosis
The falling incidence of mitral stenosis parallels that of acute rheumatic fever. Rheumatic scarring and inflammation of the valve and subvalvular tissues eventually lead to progressive fibrosis, calcification and stenosis, which usually becomes haemodynamically significant in the fourth and fifth decades.
The major cause of mitral stenosis worldwide is rheumatic fever, usually developing within a few years of acquiring the disease. In contrast, congenital abnormalities of the valve make up a larger proportion of cases in industrialized nations. The stenotic valve is the result of fusion, thickening and reduced mobility of the mitral leaflets and chordae tendinae. Valve area decreases from the normal of around 4 cm2 to less than 1 cm2, producing a diastolic gradient between the left atrium and left ventricle, raised left atrial pressure (and consequent enlargement), secondary pulmonary hypertension and right heart failure. The primary symptom is breathlessness on exertion.
Mitral Regurgitation
The most common cause of mitral regurgitation in the elderly UK population is myxomatous degeneration. The prevalence appears to be ∼2% with a 5:1 male preponderance and an even age distribution. It mainly affects the posterior mitral valve leaflet to cause chordal stretching (and ultimately rupture).
Mitral regurgitation is also a common accompaniment to ischaemic heart disease as a result of ventricular dilatation and papillary muscle dysfunction; ∼50% of patients have some degree of mitral regurgitation 1 month after suffering a myocardial infarction. Acute papillary muscle rupture may complicate myocardial infarction to cause acute mitral regurgitation, which may also result from infective endocarditis or dehiscence of a prosthetic valve.
Mitral annular calcification is a common echocardiographic finding which occurs more often in women and affects 8.5–10% of the population above the age of 50 years (although affected individuals are usually much older). In one-third there is associated mitral regurgitation, but this is usually mild and asymptomatic; accompanying mitral stenosis is rare. Other complications are unusual, although there is an associated t2–3-fold increase in the incidence of embolic stroke. Mitral valve prolapse is also frequently detected by echocardiography, although diagnostic criteria vary. Its clinical relevance is often uncertain although in some cases it may be the antecedent of a ‘floppy valve’ where the chordae are grossly elongated, the valve voluminous and mitral regurgitation more significant.
Pulmonary/Tricuspid Valve Disease
The elderly population rarely presents with primary disease of these valves and there are no meaningful epidemiological data available. Pulmonary hypertension associated with mitral valve disease can cause regurgitation of either or both right heart valves. Tricuspid stenosis may be associated with rheumatic mitral and aortic valve disease and is occasionally seen as a feature of carcinoid syndrome.
History and Clinical Assessment
Despite technological advances in the investigation and management of cardiovascular disease, an accurate history and careful clinical assessment remain the most important part of diagnosis and management in the elderly. Questioning may be more difficult and it is often necessary to enlist the help of a relative or other carer. This history should seek a background or rheumatic fever, ischaemic heart disease or hypertension and incorporate specific enquiry for symptoms of dyspnoea (either on exertion or at rest, particularly when lying flat) and exertional angina or syncope. However, symptoms in the elderly are often vague and difficult to interpret, particularly if there is concomitant pulmonary or cerebral disease.
The principles of examination are the same as those for any other age group and the weight of the patient and a dental inspection should be routinely included. However, in elderly patients many of the classical manifestations of VHD may be attenuated by the effects of ageing on the cardiovascular system, namely atherosclerosis, hypertension and valve calcification. Thus, for example, a pulse of normal character or the presence of systemic hypertension do not exclude significant aortic stenosis in the elderly. Conversely, a wide pulse pressure is not necessarily indicative of aortic regurgitation. Inspection of the jugular venous pressure is of particular importance as dependent oedema secondary to chronic venous insufficiency is common and may erroneously suggest circulatory overload. Systolic murmurs are common in the elderly and have been reported in up to 60% of normal subjects. They are frequently due to increasing rigidity and calcification of the aortic cusps without significant obstruction or mild mitral regurgitation through a floppy valve. Diastolic murmurs are always abnormal and usually indicate aortic regurgitation or mitral stenosis, although the customary accompanying opening snap in mitral stenosis may be absent in the elderly due to valve calcification.
Investigations
Electrocardiogram (ECG)
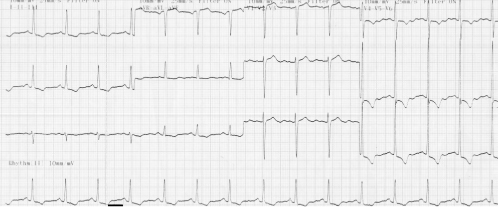
This provides important information regarding heart rate and rhythm. The ECG has low sensitivity for the detection of atrial or ventricular hypertrophy and therefore assessment of the severity of valve lesions from the ECG should be cautious (Figure 116.3). Similarly, ST/T wave changes may result from left ventricular hypertrophy or the use of digoxin and do not necessarily indicate the presence of coexisting coronary artery disease.
Figure 116.3 ECG demonstrating left ventricular hypertrophy: increased amplitude of the QRS complex, ST-segment depression and T-wave inversion in the lateral and inferior leads.
Chest X-Ray
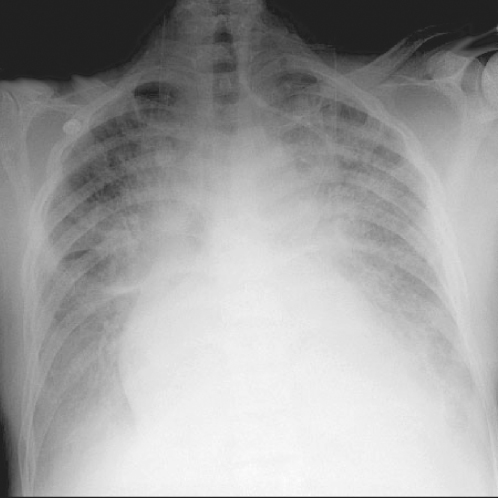
A chest X-ray is useful for the assessment of heart size, the identification of specific chamber enlargement (particularly the left atrium) or aortic dilatation and for detection of pulmonary vascular changes or oedema (Figure 116.4). It can also assist in localizing valve calcification and prosthetic valves, particularly if a lateral radiograph is requested. Finally, the detection of coincident pulmonary disease may be of considerable clinical importance in some cases, especially if invasive investigation is being considered. The cardiothoracic ratio may increase to 60% as part of normal ageing, particularly beyond 75 years, caused by a small increase in cardiac diameter and a larger fall in chest diameter. Hence the use of absolute dimensions may be more appropriate in this age group: a transverse cardiac diameter >15 cm usually indicates significant enlargement.
Figure 116.4 Chest X-ray demonstrating pulmonary oedema: cardiomegaly, alveolar shadowing, upper lobe diversion and blunting of the costophrenic angles.
Echocardiography
This is now the technique of choice for the diagnosis and evaluation of VHD and has negated the need for routine cardiac catheterization in many patients. It provides information regarding valve anatomy and physiology in addition to the effect of valve abnormality on cardiac function and allows serial observation of the patient over time. Interpretation can be difficult and requires experience, particularly in the Doppler assessment of valve stenosis when poor signal alignment may lead to underestimation of the peak velocity. Furthermore, it is a sensitive tool and ‘physiological’ regurgitation is often detected in normal subjects.
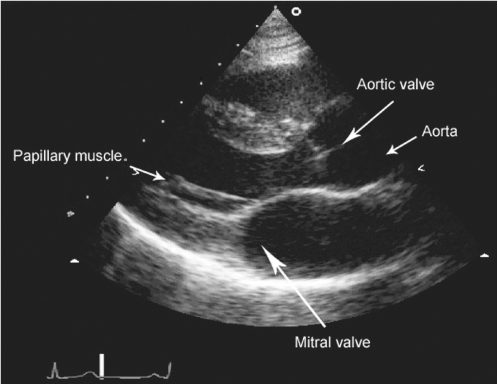
There are three principal modalities: M (or motion)-mode, two-dimensional (or cross-sectional) and Doppler echocardiography. M-mode employs a single stationary beam of ultrasound to produce a recording of motion along a single line through the heart and provides a large amount of accurate information allowing the determination of cavity dimensions and wall thickness and physiological measurements of wall motion. Two-dimensional echocardiography images a plane through the heart by sweeping a beam of ultrasound though the sector of interest and is the modality of choice for displaying anatomy (Figure 116.5). Standardized precordial views are employed and a further subcostal view may be invaluable in elderly patients, in whom obesity, lung disease, chest wall deformities or kyphoscoliosis may preclude conventional imaging. Doppler echocardiography permits accurate assessment of the position, direction and velocity of the blood flow within the heart. This information can be anatomically correlated by superimposing a colour flow Doppler map onto the two-dimensional image. Three-dimensional imaging is under development and allows reconstruction of cardiac anatomy, which may be of particular value when percutaneous intervention or surgery is being considered.
Figure 116.5 Parasternal long-axis TTE demonstrating the left ventricular cavity and mitral and aortic valves.
Transoesophageal echocardiography is a specialized technique which provides excellent image quality in almost every patient. The procedure is tolerated well by the elderly and may be performed on an outpatient basis, usually under light sedation. It has specific indications in the assessment and management of VHD and is highly sensitive in the detection of thrombus, vegetations and abscesses which may not be apparent on precordial imaging.
Cardiac Catheterization
The increasing sophistication of echocardiography means that invasive investigations are unnecessary in many patients. Nevertheless, specific questions may remain, most commonly the presence or absence of coronary artery disease in patients being considered for valve surgery. The mortality of the procedure is 0.1–0.2% and other complications (cardiac or vascular trauma, embolism, arrhythmias or contrast reactions) occur in ∼1% of cases. Patients at higher risk include the elderly and those with poor left ventricular function, severe aortic stenosis, pulmonary hypertension or peripheral vascular disease. As much information as possible should be obtained using non-invasive techniques to ensure that any invasive investigation is not unnecessarily prolonged. Furthermore, in the emergency setting, coronary angiography should not delay a potentially life-saving operation, such as emergency aortic valve replacement. In these situations, the investigations required should be decided by consultation between the physician and the surgeon and, if necessary, performed on the way to the operating theatre.
Cardiac Magnetic Resonance
Cardiac magnetic resonance (CMR) is becoming more frequently used in the assessment of VHD. It provides quantitative and reproducible measures of both stenosis and regurgitation, and also accurate measurement of the volume and function of both ventricles (Figure 116.6). A unique feature of CMR is the ability to measure flow through an image slice, with good agreement between invasive measurements and in vitro testing, allowing the quantification of regurgitation rather than a qualitative assessment of severity. The aortic root and ascending aorta are also accurately assessed and are important aspects in aortic valve disease. CMR can thus provide a comprehensive and accurate evaluation of the severity of VHD and the consequences for the ventricles. Detailed information on valve leaflet anatomy is less well assessed, however, and transoesophageal echocardiography provides higher resolution imaging.
Figure 116.6 Cardiac magnetic resonance imaging in aortic stenosis. (a) A narrow high-velocity jet in the left ventricular outflow tract; (b) ‘en-face’ view of a stenosed trileaflet aortic valve; (c) ‘en-face’ view of a stenosed bicuspid aortic valve.
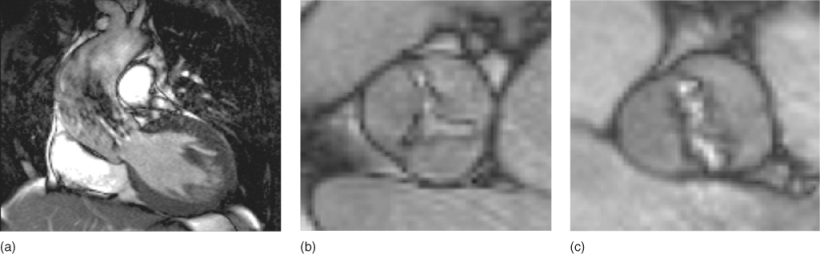
Measurement of forward and reverse flow across the aortic or pulmonary valves allows the quantification of aortic/pulmonary regurgitation, providing both regurgitant volume and fraction. The high mobility of the mitral and tricuspid valves and turbulent flow across them makes direct flow measurement difficult. In valve stenosis, valve area can be assessed by direct planimetry rather than by calculation and usually provides a better assessment of severity with CMR than velocity across the valve—velocity measurements lack the spatial and temporal resolution of Doppler echocardiography and may underestimate valve severity. Left ventricular mass can also be measured accurately, in addition to volumes and function.
At present, the limited availability of CMR restricts its use in VHD and it has a relatively high cost compared with echocardiography. Acquisition and analysis times are also longer, making it less attractive for outpatient use, although developments in scanners and software technology are likely. Arrhythmias (e.g. atrial fibrillation) may impair image quality and affect the accuracy of flow measurements, although newer imaging sequences can cope with this common problem. Claustrophobic patients can often be scanned by experienced personnel, though about 1–2% of patients may find this too difficult. CMR remains mostly contraindicated in the presence of certain ferromagnetic implants, including cerebral aneurysm clips and cardiac pacemakers/defibrillators. However, prosthetic (including metallic) valves and coronary stents are almost never a problem.
Cardiac Computed Tomography
Although cardiac computed tomography (CT) is increasingly used in the context of coronary artery disease, its use in VHD is limited. It can provide morphological information on the valves and their associated structures (particularly calcification) and also volume and mass measurements. However, it does not provide haemodynamic information, is sensitive to arrhythmias and requires a slow heart rate (often necessitating beta-blockade or other rate-slowing medication). CT also involves ionizing radiation, although newer scanners utilize techniques to reduce the dose significantly. A major future advantage of CT may be the non-invasive exclusion of coronary artery disease prior to valve surgery.
Aortic Stenosis
Symptoms and Signs
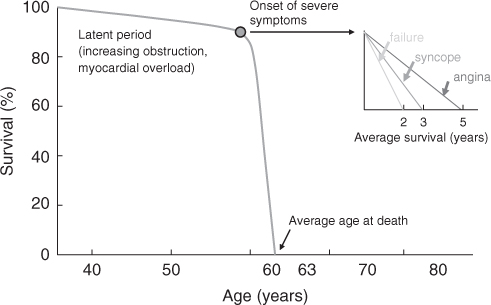
The normal aortic valve area is 3–4 cm2 and symptoms of haemodynamic origin, namely syncope, angina and dyspnoea, usually develop when this falls to 1–1.5 cm2. The onset of symptoms heralds a poor prognosis (Figure 116.7) with a 1 year mortality of 43%. In the elderly, coexisting cerebral or coronary artery disease may make interpretation difficult. Furthermore, many patients with severe aortic stenosis may be asymptomatic and the diagnosis is easily overlooked.
Figure 116.7 The natural history of aortic stenosis demonstrating a long asymptomatic preclinical phase followed by rapid decline associated with poor prognosis following the onset of symptoms.
Syncope, usually associated with exercise, becomes increasingly likely as disease progresses and is associated with an increased incidence of sudden death. The underlying mechanism is probably related to inappropriate vasodilatation secondary to abnormal baroreceptor sensitivity stimulated by elevated left ventricular pressure. In the presence of outflow obstruction, the compensatory increase in cardiac output is limited and cerebral hypoperfusion results.
Angina occurs in two-thirds of patients with aortic stenosis. Contributing factors include myocardial hypertrophy, prolonged systole and high left ventricular pressure, and these are compounded if there is coexisting coronary artery disease. The incidence of associated coronary artery disease increases with age and the need for concomitant coronary artery bypass grafting at the time of aortic valve replacement rises from 20% at 70 years to more than 60% at ages >80 years.
The onset of dyspnoea indicates significant left ventricular impairment. This is associated with a very poor prognosis and is an indication for urgent surgical intervention.
The classical peripheral manifestations of aortic stenosis (a plateau pulse and narrow pulse pressure) are often absent in elderly patients because the pulse waveform is distorted by stiff, atherosclerotic vessels. A normal or elevated blood pressure does not exclude the diagnosis. In many cases, the only physical sign is the presence of a mid systolic murmur which is usually squeaking or musical in character, loudest at the base of the heart, and typically radiates into the neck. In practice, the intensity of the murmur is a poor guide to the severity of the stenosis and in elderly patients its position is often atypical (frequently maximal at the apex). There may be an accompanying thrill and the apex beat is forceful and sustained. The first heart sound is usually normal and an ejection click (associated with a mobile valve) is uncommon in older patients. The aortic component of the second sound may be diminished (or absent) and delayed because of reduced valve mobility and the prolonged ejection time.
The finding of a systolic murmur in conjunction with any of the above symptoms should not be dismissed without further assessment. Given the high prevalence of aortic valve disease in the elderly, the poor prognosis of symptomatic aortic stenosis, the benefits of valve replacement and the difficulty of making precise clinical diagnosis, such patients require echocardiography.
Investigations
The ECG and Chest X-Ray
A normal ECG and chest X-ray do not exclude the diagnosis. The ECG usually shows left ventricular hypertrophy with repolarization changes; anteroseptal Q waves, occurring most often when there is left-axis deviation, are not unusual and may be mistakenly ascribed to a previous myocardial infarction. Occasionally, conducting tissue calcification can cause atrioventricular or bundle branch block. In the elderly, calcification of the stenosed aortic valve is invariably present on the chest X-ray, particularly in the lateral view. There may be post-stenotic aortic dilatation and cardiac enlargement and pulmonary oedema develop in the late stages.
Echocardiography
Two-dimensional and Doppler echocardiography are the techniques of choice for the assessment of suspected aortic stenosis. Aortic valve thickening and calcification are common in the elderly and associated restriction of cusp mobility is required to confirm the diagnosis. In practice, heavy calcification often obscures visualization of the valve structure and disease severity can only be determined by continuous-wave Doppler echocardiography. Echocardiography also provides the most convenient assessment of ventricular hypertrophy, size and function.
The velocity (V) of blood flow across the valve can be measured by continuous-wave Doppler echocardiography and, using the modified Bernoulli equation, the peak instantaneous pressure gradient can be derived (gradient = 4V2). Generally, there is an excellent correlation between the gradients measured using invasive and non-invasive techniques although the peak-to-peak instantaneous gradient is usually higher than the peak-to-peak gradient measured at cardiac catheterization. A Doppler gradient >70–80 mmHg (equivalent to a catheter gradient of 50 mmHg) indicates significant aortic stenosis (Figure 116.8).
Figure 116.8 Continuous-wave Doppler echocardiography in severe aortic stenosis—the peak velocity is nearly 6 m s–1 (equivalent to a trans-valve pressure gradient of ∼140 mmHg).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







