Vaginal Cancer
Primary vaginal cancer is a rare malignancy, constituting 1% to 2% of all gynecologic malignancies. According to the American Cancer Society estimates for 2010, there were approximately 2,300 new cases and 780 deaths from this disease.1 The majority of malignant lesions in the vagina are metastatic from other gynecologic malignancies or involve direct extension from adjacent sites, which excludes diagnosis as a primary vaginal malignancy. According to the staging system set by the International Federation of Obstetrics and Gynecology (FIGO), a diagnosis of primary vaginal cancer excludes any tumors involving the cervix or vulva.2 According to one study of 141 vaginal carcinoma cases, only 26% met the criteria of being a primary vaginal cancer,3 defined as a lesion that arises in the vagina without involving the cervix or vulva.
The majority of primary vaginal malignancies are squamous cell carcinomas (SCC). According to a National Cancer Data Base (NCDB) report4 based on 4,885 patients with primary vaginal cancer registered from 1985 to 1994, approximately 92% of patients were diagnosed with in situ or invasive SCC or adenocarcinomas, 4% with melanomas, 3% with sarcomas, and 1% with other or unspecified types of cancer. Sixty-six percent of all vaginal cancers were invasive, with SCC representing 79% of all invasive cases.
The peak incidence of primary vaginal cancer is in the sixth and seventh decades of life. According to data from the Surveillance, Epidemiology, and End Results (SEER) program,1 2,149 women in the United States were diagnosed with primary vaginal cancer from 1990 to 2004. The mean age at diagnosis was 65.7 ± 14.3 years and incidence rates increased with age. Vaginal cancer incidence is increasing in younger women, possibly due to an increase in human papilloma virus (HPV) infection or other sexually transmitted diseases. However, there has been an overall decrease in the incidence of primary vaginal tumors, possibly attributable to earlier detection and to implementation of strict exclusion criteria in the FIGO staging system. At the same time, there has been a steady increase in the diagnosis of vaginal intraepithelial neoplasia (VAIN) over the past several decades, due to expanded cytologic screening and increased awareness.5 Due to infrequent presentation, treatment recommendations are based on results from relatively small retrospective series, the majority of which are based on heterogeneous patient populations and treatments.
 ANATOMY
ANATOMY
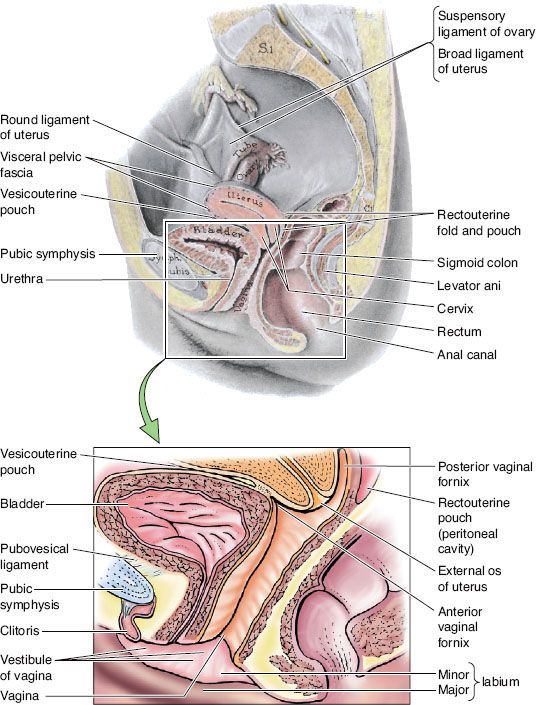
The vagina is a fibromuscular tube that extends from the cervix down to the vestibule, or cleft, between the labia minora (Fig. 72.1). It lies dorsal to the urethra and bladder base and ventral to the rectum. Superiorly, it joins the uterine cervix at an angle and, as a result, the posterior vaginal wall is longer than the anterior wall, with an overall average length of 7.5 cm. The upper aspect of the posterior vaginal wall is separated from the rectum by a reflection of peritoneum, the pouch of Douglas. The cervix projects into the upper lumen of the vagina, creating invaginations between the vaginal mucosa and the cervix, which are termed the anterior, posterior, and lateral fornices. Inferiorly, the vagina extends through the urogenital diaphragm and lies directly adjacent to the rectum up to where the fibromuscular perineal body tissue separates the vagina from the anal canal. Laterally, the vagina is adjacent to the pelvic fascia and levator ani muscles. At the introitus, the vagina has a perforated fold of thin connective tissue and mucous membrane known as the hymen.
The vaginal wall is composed of three layers: the mucosa, muscularis, and adventitia. The inner lining of the vagina is formed by a nonkeratinizing stratified squamous epithelium overlying a basement membrane with many papillae. The epithelium lacks glandular structures and instead receives lubrication from mucous secretions originating in the cervix. Underneath the mucosa is connective tissue composed of elastin and a thick muscularis layer composed of two layers of smooth muscle. The inner layer is arranged circularly, whereas the outer layer is arranged longitudinally. This muscular layer is covered by a thin adventitia that merges with neighboring organs. At the vaginal introitus, skeletal muscle forms a sphincter.
Proximally, the vagina is supplied by the vaginal artery, which arises from the cervical branch of the uterine artery and runs lateral to the vagina until it anastomoses with the inferior vesical and middle rectal arteries. The venous plexus runs parallel to the arteries, draining into the internal iliac vein. The vaginal vault is innervated by the lumbar plexus and pudendal nerve, with branches from sacral roots 2 to 4.6
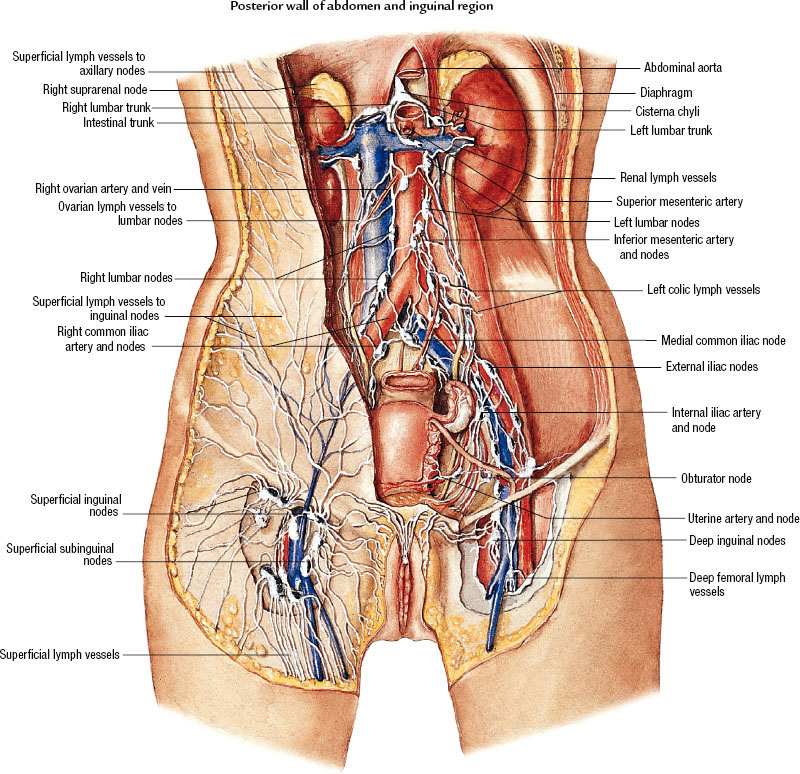
The vagina has a complex, extensive network of lymphatic drainage, with vessels that course through the submucosal and muscularis layer. The uppermost portion drains primarily via cervical lymphatics. The superior anterior vagina drains along cervical channels to the interiliac and parametrial nodes, and the posterior upper vagina drains into the inferior gluteal, presacral, and anorectal nodes (Fig. 72.2). The inferior aspect of the vagina drains into the inguinal and femoral nodes and ultimately to the pelvic nodes, following drainage patterns of the vulva. Lesions in the midvagina have been shown to drain either way.7 Lesions infiltrating the rectovaginal septum may spread to the pararectal and presacral nodes. There are multiple interconnections between lymphatic channels, and pattern of drainage cannot be reliably predicted based on location of the primary tumor. Embryologically, the vagina is believed to be of dual origin, with the upper third derived from the uterine canal, while the lower two-thirds are derived from the urogenital sinus.8
 EPIDEMIOLOGY, PRESENTATION, AND GENERAL MANAGEMENT
EPIDEMIOLOGY, PRESENTATION, AND GENERAL MANAGEMENT
Vaginal Intraepithelial Neoplasia
Epidemiology
Incidence of VAIN is estimated to be 0.2 to 0.3 cases per 100,000, with peak incidence between 40 and 60 years of age.5,9,10 Most studies do not report differences in mean age between women with low-grade and those with high-grade VAIN,11–15 although a few series have reported that patients with VAIN-1 or -2 were younger than patients with VAIN-3.16–19 Incidence of in situ vaginal cancer is estimated to be 0.1 per 100,000 women, with peak incidence between ages 70 to 79, according to data from the U.S. Centers for Disease Control and Prevention’s National Program of Cancer Registries, and the National Cancer Institute’s SEER program.20 Risk factors for VAIN include low sociocultural level, history of genital warts, hysterectomy at an early age, history of cervical intraepithelial neoplasia, immunosuppression, prior pelvic radiation, smoking, exposure to diethylstilbestrol (DES), and history of sexually transmissible diseases (STDs) or HPV infection.15,17,21,22
The diagnosis of VAIN is associated with prior or concurrent neoplasia elsewhere in the lower genital tract. Multiple series suggest approximately 50% to 90% of patients with VAIN have concurrent or prior history of intraepithelial neoplasia or carcinoma of the cervix or vulva.9,16,17 Immunosuppression from human immunodeficiency virus (HIV) is also a risk factor for both VAIN and HPV, although a higher incidence of invasive vaginal cancer in infected women has not been demonstrated.23–25 The role of pelvic radiation in the development of secondary vaginal neoplasia is unclear, with conflicting data suggesting a history of ionizing radiation may predispose to VAIN or vaginal cancer after a latency period of many years.12,26,27 In utero exposure to DES may double the risk of VAIN, thought to be due to transformation zone enlargement, increasing the risk of HPV infection.28
FIGURE 72.1. Median section of the female pelvis. The vagina is a fibromuscular tube situated posterior to the bladder and urethra and anterior to the rectum. The anterior and posterior fornices are formed by protrusion of the cervix into the vaginal canal. (From Moore KL. Clinical oriented anatomy, 4th ed. Baltimore: Lippincott Williams & Wilkins, 1999, with permission.)
Natural History
Although the likelihood of VAIN progressing to invasive disease is not fully understood, several clinical series have demonstrated a significant increase in risk of invasive vaginal cancer after a diagnosis of VAIN.12,16,29,30 Similar risk factors for VAIN and invasive vaginal cancer, as well as the younger average age at presentation of VAIN compared with invasive disease, add support to the theory that VAIN may be a precursor lesion to invasive SCC. In one series, 23 patients with VAIN, with a mean age of 41 years, were followed for at least 3 years without treatment;16 this included multifocal lesions as well as lesions associated with cervical intraepithelial neoplasia (CIN) or vulvar dysplasia. Two cases (9%) progressed to invasive cancer; one patient had VAIN-1 and progressed to stage I vaginal carcinoma in 5 years, and the second patient had VAIN-3 and progressed to stage I vaginal carcinoma in 4 years. The overall spontaneous regression rate was 78%, with the majority (78%) occurring in patients with VAIN-1 or -2. Similarly, several additional studies have demonstrated a range of 2% to 20% of patients with VAIN progressing to invasive vaginal cancer.12,16,29,31–33
The rate of occult invasive disease in patients with VAIN-3 has been reported to be as high as 28%.34 The risk of malignant transformation in VAIN-1 and -2 is less clearly elucidated; there have been reports of patients with low-grade VAIN subsequently developing invasive vaginal carcinoma.14,15
FIGURE 72.2. Lymphatic drainage of the vagina to the inguinal and pelvic lymph nodes. (Asset provided by the Anatomical Chart Company.)
Pathology
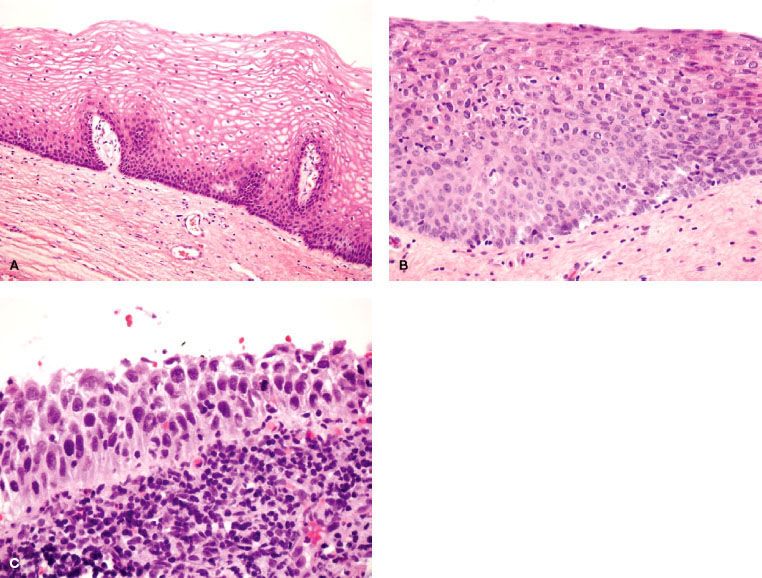
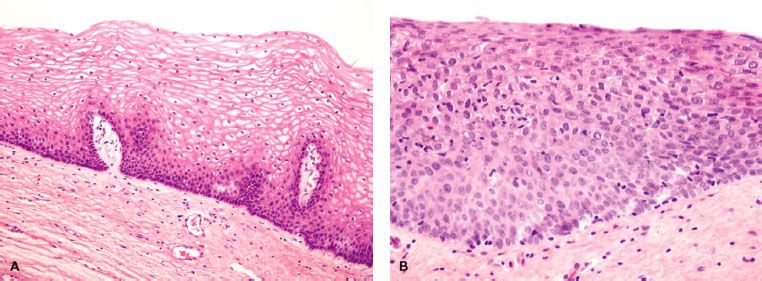
VAIN is defined as the presence of squamous cell atypia without evidence of invasion (Fig. 72.3). VAIN is further classified according to depth of epithelial involvement, with involvement of the lower one-third, two-thirds, and greater than two-thirds of the epithelium classified as VAIN-1, -2 and -3, respectively. Carcinoma in situ encompasses the full epithelial thickness and is included under VAIN-3. Excluded from diagnosis of VAIN is the presence of glandular intraepithelial dysplasia or atypical vaginal adenosis; these entities are associated with in utero DES exposure and are deemed to be precursors of DES-associated clear cell adenocarcinoma.35 VAIN is frequently multifocal and most commonly involves the upper portion of the vagina.
Histopathologically, most lesions are epidermoid and exhibit full-thickness alterations with atypical mitoses and hyperchromatism (Fig. 72.3).36 Punctation and mosaic patterns are often noted with high-grade VAIN.14 Most lesions are multifocal and can involve all surfaces of the vagina, although the superior one-third of the vagina is most common.12,16
VAIN is associated with HPV infection.37 A review of 232 published VAIN cases documented a high prevalence of HPV using polymerase chain reaction or hybrid capture assays for detection, with 98.5% and 92.6% of VAIN-1 and VAIN-2 or -3 cases positive for HPV.38 A series by Sugase and Matsukura39 examining 71 biopsy specimens of VAIN found HPV in 100% of samples. Fifteen different known subtypes were identified (HPV-16, -18, -30, -31, -35, -40, -42, -43, -51, -52, -53, -54, -56, -58, -66). Types HPV-16 or -18 comprised 9%, 7%, and 67% of VAIN-1, -2, and -3 cases, respectively.
FIGURE 72.3. Normal vaginal epithelium (A), vaginal intraepithelial neoplasia (VAIN)-2 (B) and VAIN-3 (C). Compared with normal vaginal mucosa, VAIN lesions display architectural and cytologic abnormalities, such as nuclear hyperchromasia, pleomorphism, undifferentiated cells scattered within the epithelium, and cellular crowding. In VAIN-3, dysplastic cells involve the full epithelial thickness without stromal invasion. (Courtesy of Marisa R. Nucci and Carlos Parra-Herran.)
Clinical Presentation
VAIN is usually asymptomatic12 and most commonly detected after cytologic evaluation as part of surveillance in patients with a history of CIN or invasive cervical carcinoma. According to the American Cancer Society guidelines from 2002, surveillance cytology for VAIN in posthysterectomy patients is recommended if there is a history of cervical pathology.40 However, evidence does not support routine surveillance in patients without a history of CIN or invasive cervical cancer.
Prognostic Factors
A study by So et al.41 on 48 women with VAIN reports a significant association between higher viral load of HPV and the likelihood of persistent disease after treatment. There is also an association between a history of pelvic radiation and development of VAIN,33,42,43 with up to 20% of patients with prior radiation developing vaginal dysplasia. A retrospective review of 33 patients with VAIN treated at the University of Pennsylvania found patients with a history of radiation therapy to be more refractory to treatment, with a significantly higher likelihood of recurrence after surgical and ablative therapy.31 Patients with a history of radiation had an odds ratio of 3.6 for recurrent disease (95% confidence interval, 1.5 to 9.0) compared with patients without a history of radiation.
Treatment Options
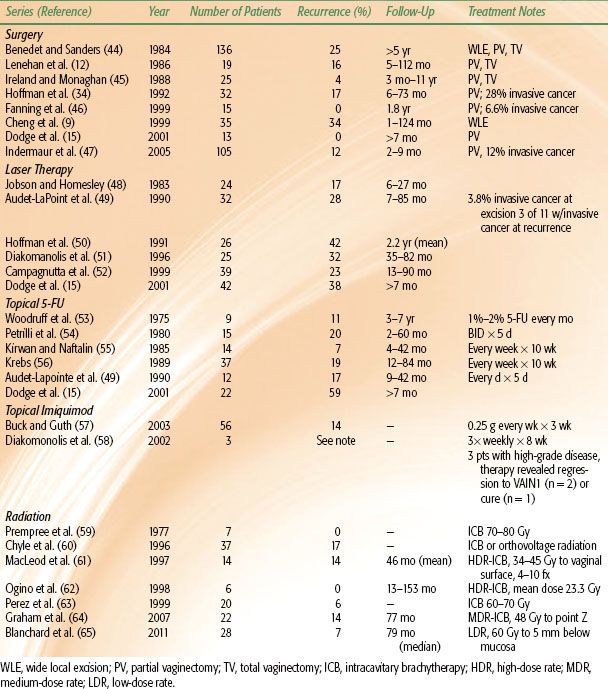
The management of VAIN is heterogeneous, with a wide variety of treatments available. There is currently no consensus on optimal treatment modality, as reported data are generally retrospective and based on decades of experience with varied treatments and patient characteristics; thus it is difficult to compare different treatment modalities (Table 72.1). Treatment approaches include local excision, partial or total vaginectomy, laser vaporization, electrocoagulation, topical 5% fluorouracil (5-FU) administration, and radiation.14,15,17–19,47,54,64,66,67 Reported success rates for different approaches range from 48% to 100% for laser vaporization,50,68,69 52% to 100% for colpectomy,34,47,51 75% to 100% for topical 5-FU,53,54,55,56,70,71,72,73 and 83% to 100% for radiation.61,62,65,67,74 Given the breadth of available therapies, an individualized approach to patient management is advised, with consideration given to the patient’s overall health, desire to preserve sexual function, candidacy for surgery, disease multifocality, and prior treatment failures.
Most patients with VAIN-1 are offered close surveillance. Lesions often regress spontaneously; in one study by Aho et al.,16 78% of patients with VAIN-1 or -2 had spontaneous regression of disease without treatment. Appropriate treatment for VAIN-2 should be determined on an individual basis, based on disease extent and associated patient factors. Therapy for VAIN-3 should be more aggressive, as there is a higher likelihood of progression to invasive disease, including occult invasive disease.34,47
TABLE 72.1 LOCAL CONTROL OF VAGINAL INTRAEPITHELIAL NEOPLASIA BY TREATMENT MODALITY
Surgical and Ablative Therapies
Surgical approaches include local excision, partial vaginectomy, and, in rare cases, total vaginectomy for highly extensive disease, which provides the advantage of obtaining a complete pathologic diagnosis. Most resections can be performed through a transvaginal approach. Location of VAIN in the vaginal vault or posthysterectomy suture recesses may require partial vaginectomy for complete resection.
Local therapy is achieved through a cold-knife approach, electrosurgical loop excision, laser, or via ultrasonic surgical aspiration.75–77 The carbon dioxide laser has been used for ablation of local tissue, with multiple treatments required in approximately one-third of patients.49,50,52,56,69,78,79 Complications include postoperative pain, scarring, and bleeding; however, the treatment is overall fairly well tolerated, with minimum impact on sexual function.80 Diakomanolis et al.51 reported on 52 patients who underwent laser treatment or partial vaginectomy and found results to favor laser ablation for multifocal disease and partial vaginectomy for unifocal disease. Ultrasonic surgical aspiration is another technique that has shown efficacy similar that of to laser ablation; in one series of 110 patients, 1-year recurrence-free survival rates were 24% and 26%, respectively.47
Series on surgical treatment of VAIN report recurrence rates in the range of 0% to 50%, with follow-up times ranging from 3 months to 18 years.9,12,17,34,44 Overall, series looking specifically at upper vaginectomy report control rates of 68% to 88%.19,29,34,47,51 For example, Hoffman et al.34 reported that 83% of patients with VAIN-3 remained free of disease with a mean follow-up time of 38 months. Of note, 28% of all patients were found to have occult invasive disease upon upper vaginectomy. A subsequent study by Indermaur et al.,47 which retrospectively reviewed 36 patients treated with upper vaginectomy for VAIN, reported 88% to be free of recurrence with a mean follow-up time of 25 months. Thirteen patients (12%) were found to have invasive cancer; 8 of 13 had frank invasive disease, while 5 patients had microinvasive carcinoma. Complication rates of upper vaginectomy have been variably reported; in the series by Indermaur et al.,47 there was a 9% complication rate. Potential complications from surgery depend on the extent and method of surgical resection, and they range from vaginal shortening and stenosis to standard postoperative morbidity associated with abdominal procedures. It should be noted that patients with a history of radiation treatment are at higher risk of postoperative complications, with a higher rate of fistula formation reported in one study.9
Topical Treatments
Topical therapies have been utilized in patients with early-stage lesions, multifocal disease, or multiple comorbidities, rendering them nonideal surgical candidates. Topical applications have also been utilized prior to surgery to reduce lesion size and improve stripping of neoplastic epithelial cells from underlying stroma.17 Treatments include topical 5-FU and 5% imiquimod cream, with response rates in 71% to 78% of patients for imiquimod and 41% to 88% for 5-FU.53,55,56,57,58,67,71,72,81,82 Imiquimod increases levels of interferon-alfa, interleukin-12, and tumor necrosis factor,58 resulting in immunomodulation of the vaginal mucosa. Side effects of topical treatments include local irritation, with burning and ulceration being the most commonly reported adverse events.53,71
Radiation Therapy
Radiation therapy is an alternate treatment with a long history of efficacy, with several small series over the past 20 to 30 years reporting control rates ranging from 80% to 100%.12,18,49,62,64, 65,67,74,83,84 High-dose-rate (HDR), medium-dose-rate (MDR), and low-dose-rate (LDR) techniques have been reported with acceptable results, although it is difficult to compare regimens due to small patient numbers, generally short follow-up times, and overall nonuniformity among series. Generally, radiation is reserved for patients who relapse after more conservative treatments. Drawbacks to radiation include potential undertreatment of occult invasive disease, the risk of secondary malignancy, and long-term morbidity, although there are no prospective data available regarding the impact of treatment on sexual function and quality of life.
LDR treatment is most commonly delivered with an intracavitary vaginal cylinder using cesium-137. Typically, a dose of 60 Gy is prescribed to the vaginal mucosa, but a wide range of doses, depending on depth of dose prescription, as well as a variety of techniques, have been reported.63,65,67,74,84 Chyle et al.60 prescribed 70 to 80 Gy to the vaginal surface and reported a 17% recurrence rate at 10 years in their series of 37 patients. Perez et al.63 treated patients to the vaginal surface with a dose of 60 to 70 Gy, and reported 1 recurrence in 20 patients. The recurrence occurred in the distal vagina and was noted to be a marginal recurrence. Blanchard et al.65 reported on a series of 28 patients with VAIN-3 treated at Institut Gustave Roussy from 1985 to 2008. Patients were treated with LDR brachytherapy, using a vaginal mold technique, to a dose of 60 Gy prescribed 5 mm below the vaginal surface; 18 patients received treatment to the upper half of the vagina, 6 were treated to the upper two-thirds, and 4 were treated to the whole vaginal length. With a median follow-up time of 41 months, the authors report only one in-field recurrence, with a 10-year local control rate of 93%. Treatment with LDR brachytherapy is overall well tolerated; in the Blanchard et al.65 series, there were no grade 3 or 4 late toxicities and only one grade 2 gastrointestinal toxicity noted. This is consistent with the Perez et al.63 series, in which there was only one grade 3 urinary complication among 40 patients with VAIN-3 or stage I vaginal cancer treated with LDR. Overall, excellent local control and low toxicity have been reported for LDR brachytherapy.
Graham et al.64 reviewed their experience using MDR intracavitary brachytherapy for VAIN-3 at the Beatson Oncology Centre in Glasgow, UK. Using a MDR Selectron (Nucletron, Holland), 48 Gy was prescribed 0.5 cm lateral to the ovoid surface (point Z) over two insertions, spaced 1 week apart. Ovoids were chosen over vaginal cylinder placement in order to adequately cover epithelium sutured into the superolateral vagina at hysterectomy. With a median follow-up duration of 77 months, recurrent or residual VAIN-3 was documented in three patients, and two of these patients subsequently developed invasive or microinvasive vaginal carcinoma. One other patient developed late progression 14 years after treatment. There were minimal acute effects during treatment; however, with longer follow-up, all patients were noted to have grade 1–2 mucosal atrophy, dryness and telangiectasia. Four patients developed grade 3 toxicity with severe vaginal stenosis, and one patient developed grade 4 toxicity, with a vaginal ulcer that presented 2 years after treatment. An additional patient developed grade 3 urinary toxicity with urethral stricture requiring intermittent self-catheterization.
HDR brachytherapy has been used for patients with VAIN-3. Ogino et al.62 reported their experience treating six patients with VAIN-3 at Kanagawa Cancer Center from 1983 to 1993, with a mean dose of 23.3 Gy (range, 15 to 30 Gy); most treatments were delivered in 5 fractions using two ovoids, with dose calculated to a point 1 cm superior to the vaginal apex. Lesions distal to the vaginal vault had doses calculated 1 cm beyond the plane of the vaginal cylinder in order to deliver adequate dose to the entire vagina. Median follow-up was 90.5 months, and there was no evidence of disease recurrence in the treated patients. Two patients developed moderate to severe vaginal stenosis, and three patients developed rectal bleeding, which resolved. MacLeod et al.61 reviewed their experience treating 14 patients with VAIN-3 from 1985 to 1995. Total dose was 34 to 45 Gy to the vaginal surface, in 8.5-Gy fractions delivered twice a week or 4.5-Gy fractions delivered 4 times a week. One patient developed invasive cancer, and one patient had persistent VAIN-3. There were no major acute toxicities, and two patients developed late grade 3 vaginal atrophy and stenosis. Mock et al.83 reported treatment of six patients with HDR intracavitary brachytherapy, with 100% 5-year disease-specific survival.
Malignant Tumors of the Vagina: Squamous Cell Carcinoma
Epidemiology
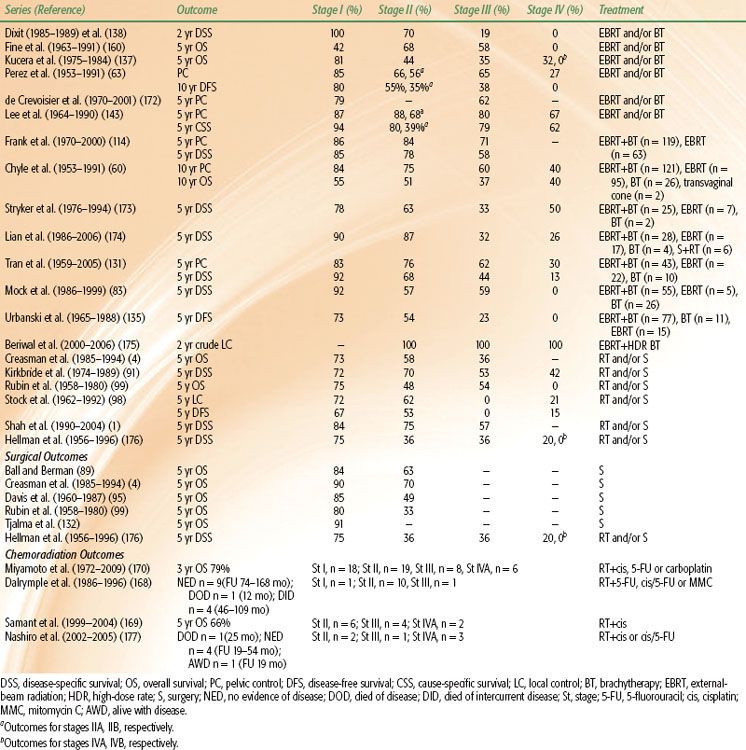
A review of five series, including a total of 1,375 cases of vaginal cancer, reported a FIGO stage distribution as follows: 26% stage I, 37% stage II, 24% stage III, and 13% stage IV.85 Consistent with these data, the NCDB review by Creasman et al.,4 for the period of 1985 to 1994, revealed 3,244 cases of invasive primary vaginal carcinoma, with 24% of patients presenting with American Joint Committee on Cancer (AJCC) stage I disease, 20% AJCC stage II, 24% AJCC stages III an IV, and 32% unknown. Most tumors were moderately (28%) or poorly (28%) differentiated at presentation.
According to the SEER study by Shah et al.,1 most women diagnosed with primary vaginal cancer are non-Hispanic whites (66%), followed by African Americans (14%), Hispanic whites (12%), Asian/Pacific Islanders (7%), and others (1%). Incidence rates were highest for African American women (1.24/100,000 person-years) and lowest for Asian/Pacific Islanders (0.64/100,000 person-years). The greatest proportion of women (36%) presented with stage I disease, and 65% had squamous histology, consistent with other reports.
Risk Factors
Primary vaginal SCC shares similar risk factors with VAIN and, in general, with cervical neoplasia. Potential risk factors for SCC include HPV infection, history of CIN, vulvar intraepithelial neoplasia, immunosuppression, and possibly history of pelvic radiation, although this is controversial. In a population-based case-control study of 156 women with VAIN or invasive cancer, risk factors included early onset of intercourse, increased number of lifetime sexual partners, and current smoking. HPV DNA was detectable in 80% of patients with in situ disease and 60% of those with invasive disease, and 30% of patients reported a history of treatment for invasive malignancy, most commonly cervix or in situ anogenital neoplasia.30 A case-control study of 41 women with in situ disease or invasive carcinoma identified low socioeconomic status, history of genital warts, vaginal discharge or irritation, history of abnormal cytology, prior hysterectomy, and vaginal trauma as potential risk factors.86 A larger case-control study of 36,856 women found an increased risk of vaginal cancer in alcoholic women, likely associated with a higher incidence of lifestyle factors, such as promiscuity and smoking, which are also associated with a higher incidence of HPV infection. Early hysterectomy appears to be a risk factor in some studies, if performed for malignant or premalignant disease.30,87
Patients with a history of cervical cancer have a significantly higher risk of developing in situ as well as invasive carcinoma. Studies suggest that 10% to 50% of patients with a history of VAIN or invasive carcinoma of the vagina have undergone treatment for in situ or invasive cervical carcinoma,12,60,88–94 with the interval from treatment of cervical disease to development of vaginal carcinoma averaging approximately 14 years.90,95 HIV-infected women are also at higher risk of developing vaginal carcinoma, which tends to behave more aggressively in this setting than in HIV-negative patients.96
The role of ionizing radiation to the pelvis in the development of vaginal carcinoma is unclear, with conflicting reports. According to one study that analyzed 1,200 patients treated over a 20-year period for carcinoma of the cervix, prior radiation therapy was not shown to result in increased secondary pelvic neoplasms.27 A second study by Boice et al.,26 however, reported a 14-fold increased risk of vaginal cancer in women with a history of pelvic irradiation before the age of 45, with a significant dose–response relationship.
Other proposed causes include chronic irritation of the vaginal mucosa, resulting in chronic inflammation, hyperkeratosis, thickening, and acanthosis,96 with subsequent metaplastic and dysplastic changes. Although older studies showed that more vaginal cancers arise from the posterior vaginal wall, other studies report approximately equal distribution of invasive carcinomas on the anterior and posterior walls,29,90,97–98,99 arguing against the theory that pooling of irritating substances in the posterior fornix contributes to development of vaginal cancers, particularly on the posterior wall. Chronic irritation from use of vaginal pessaries has also been implicated as a contributor in vaginal cancer development.100,101
Clinical Presentation
Vaginal tumors can spread along the vaginal walls to involve the cervix or vulva, but involvement of the cervix or vulva at the time of diagnosis excludes classification as a primary vaginal cancer. Lesions can extend radially, either into the lumen to form exophytic masses or through the vaginal wall to invade surrounding musculature and organs. Anterior wall lesions can infiltrate the vesicovaginal septum or urethra. Posterior wall lesions can infiltrate the rectovaginal septum and involve the rectal mucosa. Advanced disease can extend laterally toward the parametrium and paracolpal tissues or into the urogenital diaphragm, levator ani muscles, or pelvic fascia, and eventually to the pelvic side wall.
Grossly, SCC of the vagina can present as nodular, ulcerated, indurated, exophytic, or endophytic lesions, and it is difficult to histologically distinguish a primary vaginal SCC from recurrent cervical or vulvar carcinoma. Histologically, tumors are graded as well, moderate, or poorly differentiated and have been described as keratinizing, nonkeratinizing, basaloid, warty, or verrucous. The majority of these lesions are nonkeratinizing and moderately differentiated (Fig. 72.4).102
Vaginal carcinoma most frequently involves the superior one-third of the vaginal canal, with series reporting 50% to 83% of cases occurring in this region.29,98,99,103–106 A high proportion of patients have a history of prior hysterectomy. There is approximately equal involvement of the middle and inferior thirds,29 although some studies suggest that involvement of the lower third is more common than involvement of the middle third.90,99 Older series report involvement of the posterior vaginal wall to be more common, although other series suggest involvement of the anterior and posterior walls occurs at equal frequencies.90,98,99 The lateral walls are less frequently involved. Tumors may exhibit an exophytic or ulcerative, infiltrating growth pattern.
HPV has been implicated in the pathogenesis of vaginal SCC. Fuste et al.107 examined histopathologic patterns of HPV infection and vaginal SCC. They did not find any association between the type of HPV and histology (keratinizing, basaloid, warty). Overall, 75% of specimens were positive for HPV. HPV-16 was identified in 72% of positive samples. Ferreira et al.108 also noted a high percentage of HPV-positive tumors, with 81% of SCC specimens positive for HPV and HPV16 found in the majority of tumors.
FIGURE 72.4. Invasive squamous cell carcinoma of the vagina at 10X (A) and 40X (B) magnification. (Courtesy of Marisa R. Nucci and Carlos Parra-Herran.)
Verrucous carcinoma is a distinct histologic variant of vaginal SCC that commonly presents as a well-circumscribed, soft, cauliflower-like mass that is microscopically well differentiated, with a papillary growth pattern and acanthotic epithelium.109 There is surface maturation with parakeratosis or hyperkeratosis without koilocytosis. This variant of SCC exhibits less aggressive behavior and rarely metastasizes.109–112 Therefore, it should be considered a distinct entity from other vaginal SCC.
Up to 65% of patients present with irregular vaginal bleeding as their primary symptom.90,113,114 Vaginal discharge is the second most common symptom, occurring in 10% to 15% of patients. Less frequent symptoms, associated with locally advanced disease, include the presence of a mass; pain; urinary symptoms, including frequency, dysuria, or hematuria; or gastrointestinal complaints such as tenesmus, constipation, or melena. Due to the proximity of anterior wall lesions to the urethra and bladder, urinary symptoms can be seen more commonly in vaginal cancer than in cervical cancer. Up to 20% of women are asymptomatic at the time of diagnosis,90,115 with lesions detected via cytologic screening or by speculum examination.
Patterns of Lymphatic Drainage
The lymphatic system of the vagina is complex, with many interconnections. Lymphatic channels in the mucosa run parallel to networks of channels in the submucosa and muscular layer, ultimately converging to form trunks at the vaginal wall periphery, which subsequently drain to major pelvic nodal groups. The upper vagina drains to the obturator and hypogastric nodes, similar to the cervix. The lower vagina drains to the inguinal, femoral, and external iliac nodes, and posteriorly situated lesions can drain to the inferior gluteal, presacral, or perirectal nodes. Due to considerable crossover drainage, the location of the primary tumor is not a reliable indicator of drainage site.
Frumovitz et al.116 utilized lymphoscintigraphy to determine patterns of lymphatic drainage in 14 women diagnosed with primary vaginal cancers and found a substantial degree of anomalous drainage, resulting in a change in radiation treatment for 33% of patients. For example, among four women with lesions located in the upper third of the vagina, which is predicted to drain along the cervical lymphatic chains to the pelvis, two (50%) were found to have a sentinel node in the inguinal region. Among five women with lesions located at the vaginal introitus, a location predicted to drain along the vulvar lymphatic chains to the inguinal triangle, three (60%) were found to have a sentinel node in the pelvis.
The risk of nodal metastasis appears to increase significantly with stage, although the true incidence of positive lymph nodes is difficult to determine because most patients receive treatment with radiation therapy and do not undergo surgical lymphadenectomy. Sparse data on nodal metastases are derived from series in which exploratory laparotomies and lymphadenectomies were performed.95 The incidence of lymph node involvement has been reported to be 0% to 14% in stage I and 21% to 32% in stage II disease.95,117,118 The incidence of nodal involvement in stages III and IV has been reported to be as high as 78% and 83%, respectively.99 At diagnosis, up to 20% of patients have clinically positive inguinal nodes, with reported ranges of 5.3% to 20%.63,93 The risk of nodal failure increases significantly with local recurrence. Chyle et al.60 reported 10-year inguinal and pelvic failure rates of 16% and 28%, respectively, in patients with local recurrence, in contrast to 2% and 4%, respectively, in patients without local recurrence.
Distant metastases can occur with advanced disease at presentation or upon recurrence after primary therapy. The most frequent site of hematogenous metastasis is the lung, with less commonly noted sites being liver and bone.60 In a series by Perez et al.,93 the incidence of distant metastasis was 16% for stage I, 31% for stage II, 46% for stage IIB, 62% for stage III, and 50% for stage IV. Some histologies may have a higher likelihood of distant metastases than others. Chyle et al.60 noted a higher incidence of distant metastases in patients with adenocarcinoma (48%) than in those with SCC (10%), with correspondingly lower 10-year survival rates (20% vs. 50%). Leiomyosarcomas are also aggressive; they undergo early hematogenous dissemination, frequently occur locally,4,119 and demonstrate frequent pulmonary metastases.120 Vaginal melanoma and neuroendocrine small cell tumors are highly malignant, and both have a propensity for early hematogenous spread.121,122
Diagnostic Workup
The diagnostic workup should start with a thorough history and physical examination, with careful attention given to the pelvis. Examination under anesthesia is recommended for complete assessment of tumor extent and assessment of vaginal walls. During speculum examination, the speculum blades can obscure the anterior and posterior walls, so it is essential to rotate the speculum for visualization of all four walls from the introitus to the apex. Bimanual examination, with careful digital palpation, should be performed.
A definitive diagnosis is achieved with biopsy of suspected lesions, which can present as an exophytic mass, plaque, or ulcer. Up to 20% of vaginal malignancies are detected incidentally as a result of cytologic surveillance.90 If a lesion is not visible in the setting of abnormal cytology, colposcopy with acetic acid, followed by Lugol’s iodine stain, is conducted. Biopsies of white epithelium or atypical vascularity should be obtained after application of acetic acid. Iodine will identify Schiller-positive regions, which are nonstaining and should correspond with areas identified following application of acetic acid. Adequate biopsies should include the cervix, if present, to rule out a cervical primary. Patients can present with multiple regions of abnormality. Inguinal nodes should be palpated for disease involvement, particularly if the primary lesion is situated in the lower portion of the vagina, as 5% to 20% of patients have been reported to have involved inguinal nodes at presentation.63,93 Suspicious nodes warrant a biopsy. Laboratory tests include a complete blood count with differential and assessment of renal and hepatic function.
FIGO staging of vaginal cancer is clinical and allows chest x-ray, intravenous pyelography (IVP), barium enema, cystoscopy, and proctosigmoidoscopy. Cystoscopy or proctosigmoidoscopy may be necessary in patients with symptoms suggestive of bladder or rectal infiltration. Computed tomographic (CT) imaging and magnetic resonance imaging (MRI) do not affect FIGO stage assignment and are commonly used. CT of the pelvis is obtained in place of IVP to assess the renal parenchyma and also to obtain information on the extent of local disease and lymph node status. MRI can provide salient treatment planning information by characterizing extent of invasion and differentiating malignant tumor, which is isointense to muscle on T1 and hyperintense on T2, from normal structures or fibrosis.123 Advantages of MRI over other imaging modalities include superior soft tissue contrast resolution, allowing accurate assessment of tumor volume and extent of local invasion, and accurate assessment of pelvic-nodal involvement. In general, MRI is regarded as superior to CT for staging of gynecologic malignancies and should be obtained when available.
Positron emission tomography (PET) has shown efficacy in detecting the extent of primary tumor and abnormal lymph nodes in vaginal cancer with higher sensitivity than CT,124 as is the case with cervical carcinoma. Primary vaginal carcinoma and metastatic lesions demonstrate avid uptake of 2-[fluorine-18]fluoro-2-deoxy-D-glucose (FDG). In one study, 23 patients with primary vaginal carcinoma received both PET and CT during staging. CT identified the primary tumor in only 43% of patients, whereas PET identified the tumor in 100%. PET identified suspicious uptake in groin and pelvic nodes in 8 of 23 patients, compared with 4 of 23 with CT. Treatment planning was modified in 14% of patients due to findings from PET, and the authors concluded that PET detects primary tumor and abnormal lymph nodes more often than CT.124 It is important that the patient have an empty bladder prior to imaging, as physiologic FDG activity in a filled bladder can potentially interfere with accurate estimation of vaginal involvement. In practice, most patients undergoing planning for radiation treatment are assessed with CT as well as MRI or PET, based on extrapolation from studies of other gynecologic malignances as well as these studies.
TABLE 72.2 AMERICAN JOINT COMMITTEE ON CANCER’S STAGING OF VAGINAL CANCER
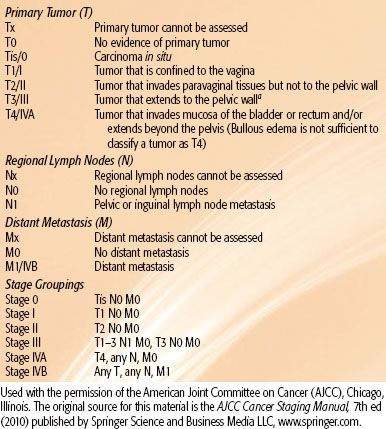
TABLE 72.3 INTERNATIONAL FEDERATION OF GYNECOLOGY AND OBSTETRICS STAGING SYSTEM FOR CARCINOMA OF THE VAGINA

Staging
The AJCC125 and FIGO2 systems are used to stage vaginal cancer (Tables 72.2 and 72.3). FIGO is a clinical staging system that allows chest x-ray, IVP, barium enema, cystoscopy, and rectosigmoidoscopy for staging purposes. Vaginal cancer is a diagnosis of exclusion, with involvement of the cervix or vulva classified as primary cervical or vulvar cancers, respectively. Primary vaginal melanomas and lymphomas are staged according to the AJCC staging systems for melanomas and lymphomas, respectively.125
For patients with a prior gynecologic malignancy, a 5-year period free of disease is generally considered adequate to allow for distinction between recurrent disease and a new primary vaginal cancer. FIGO no longer recognizes carcinoma in situ as stage 0.
Stage I disease is defined as limited to the vaginal wall, and stage II disease involves subvaginal tissue without extension to the pelvic wall. Discriminating between stages I and II can be subjective; thin tumors <0.5 cm are generally classified as stage I, with thicker infiltrating tumors or those with paravaginal nodularity classified as stage II. Perez et al.63 proposed a modification to the FIGO system in 1973, distinguishing tumors with paravaginal submucosal extension only (stage IIA) from tumors with parametrial infiltration (stage IIB). The study reported a 20% 5-year survival difference (55% vs. 35%) between stages IIA and IIB. This modification has not been adopted into FIGO staging; however, some investigators consider the distinction to be prognostically relevant.59,93
Prognostic Factors
The most significant prognostic factor is stage at time of presentation;1,63,91,126–129 the NCDB, the largest population-based series on vaginal cancer thus far, reports 5-year survival rates of 96% for stage 0, 73% for stage I, 58% for stage II, and 36% for stages III and IV disease.4 The series by Shah et al.,1 based on SEER data for women diagnosed between 1990 and 2004, also reveals the correlation between stage and outcome, with 5-year disease-specific survival rates of 84% for stage I, 75% for stage II, and 57% for stages III and IV; the adjusted hazard ratio for mortality, on multivariate analysis, was 4.67. In the Perez et al.93 series, 165 patients with primary vaginal cancer were treated with definitive radiation therapy and had 10-year actuarial disease-free survival rates of 94% for stage 0, 75% for stage I, 55% for stage IIA, 43% for stage IIB, 32% for stage III, and 0% for stage IV. Lymph node involvement also carries an unfavorable prognosis.130
Size of the initial lesion is a prognostic factor that has shown significance in several series. The SEER database study,1 which included 2,149 women with primary vaginal cancer, noted a significantly lower 5-year survival rate in women with tumors ≥4 cm than in women with tumors <4 cm (65% vs. 84%); however, size information was missing for 52% of women. After multivariate analysis, the women with the larger tumors had an adjusted hazard ratio of 1.71 for mortality. Chyle et al.,60 in their review of 301 patients treated at the MD Anderson Cancer Center (MDACC) from 1953 to 1991, found that women with lesions >5 cm in maximum diameter had a significantly higher 10-year local recurrence rate than those with smaller lesions (40% vs. 20%). The series by Hellman et al.,128 with 314 patients treated at the Karolinska University Hospital from 1956 to 1996, found only three factors to independently predict for poor survival on multivariate analysis: advanced age, tumor size ≥4 cm, and advanced stage. Tumors comprising two-thirds or more of the vagina and tumors growing circumferentially were associated with an extremely poor prognosis. The series by Tran et al.,131 which reviewed records of 78 patients with SCC treated at Stanford University Medical Center from 1959 to 2005, also found size to be a prognostic factor for disease-free survival on multivariate analysis, along with stage, prior hysterectomy, and pretreatment hemoglobin level. Smaller series by Tjalma et al.132 and Kirkbride et al.91 also describe adverse outcomes with larger tumor size. Other series have failed to show significance, but they likely were hindered by small numbers, difficulties in accurate assessment of size, and treatment heterogeneity. Frank et al.114 reviewed data on 193 patients treated at MDACC between 1970 and 2000 for vaginal SCC and found a nonsignificant difference in disease-specific survival rates between patients with tumors ≤4 cm in diameter and those with tumors >4 cm (82% vs. 60%, respectively). Extent of vaginal canal involvement has also been examined, as a surrogate for tumor size, in the assessment of tumor burden. In a series by Stock et al.,98 which examined 100 cases of primary vaginal carcinoma treated at Magee Women’s Hospital from 1962 to 1992, patients with involvement of one-third of the vaginal canal or less had a significantly higher 5-year disease-free survival rate (61%) than patients with more extensive involvement (25%).
There is conflicting evidence on the impact of lesion location on prognosis; it has been noted in some60,103,133–135 but not all93,106,136 reports. In an analysis of 110 patients by Kucera et al.,137 5-year survival rates were 60% for lesions of the upper third of the vagina, 37.5% for lesions of the middle third, and 37% for the lower third. Chyle et al.60 noted a 17% rate of pelvic relapse in patients with tumors in the upper third of the vagina, 36% for patients with tumors in the middle or lower third, and 42% for patients with whole vaginal involvement. Lesions in the posterior wall were also noted to be associated with a worse prognosis than lesions involving the anterior vaginal wall,60 with 10-year recurrence rates of 32% versus 19% on univariate analysis (<.007). The Hellman et al.128series found no difference in prognosis between anterior and posterior tumors.
Histologic grade has been found to be an independent significant predictor of survival in several series91,103,135 but not others. Hellman et al.128 evaluated the impact of tumor grade and other histopathologic variables (mitotic activity, koilocytosis, growth in vessels, lymphocytic reactions) and found no correlation with survival.
Age at diagnosis correlated significantly with poor survival in both univariate and multivariate analysis in the Hellman et al.128 series. Age was also noted to be a significant prognostic factor in the Urbanski et al.135 series, with 5-year survival rates of 83% for patients younger than 60 compared with 25% for those 60 years of age or older (P <.0001); other series have failed to demonstrate the statistical significance of age.63,138
Tran et al.131 reviewed records of 78 patients with primary SCC of the vagina treated at Stanford University Hospital and found a hemoglobin level <12.5 g/dL prior to definitive treatment to be prognostic for worse pelvic control and disease-specific survival;117 5-year disease-specific survival rates were 55% for women with hemoglobin levels <12.5 g/dL and 76% for those with levels ≥12.5 g/dL. This remained significant after multivariate analysis, along with prior hysterectomy, stage, and tumor size.
Up to 62% of patients with primary vaginal cancer have had a prior hysterectomy.139 This high rate reflects the proportion of patients with a history of cervical pathology as well as the increased hysterectomy rate in the general female population.140 The study by Tran et al.131 is the first to identify prior hysterectomy as a favorable prognostic factor on multivariate analysis. This may reflect more rigorous surveillance in posthysterectomy patients, resulting in tumors discovered at an earlier stage, or may be a reflection of less overall vaginal tissue as a substrate for tumorigenesis. Two studies have identified hysterectomy as a significant prognostic factor in univariate analysis.60,128
The prognostic role of HPV was examined by Brunner et al.141 in their series of 35 patients with primary invasive SCC of the vagina. Using in situ hybridization, HPV was detected in 51.4% of cases. There was no significant influence on clinical stage, grade, or tumor size nor did prognosis differ between HPV-positive and HPV-negative tumors. However, in a subset of patients with FIGO stage III or higher disease, HPV positivity was found to correlate with improved disease-free and overall survival (P .004 and .023, respectively). In contrast, Fuste et al.107 found a trend toward longer survival in women with HPV-positive tumors in their series of 32 patients, with median survival times of 113.9 months versus 19.7 months for women with HPV-positive and HPV-negative tumors, respectively (P = .15).
For patients treated with radiation, treatment time may be a significant factor impacting tumor control.142,143 Lee et al.143 found overall treatment time of ≤9 weeks to be associated with a pelvic tumor control rate of 97% as compared with 57% for treatment time >9 weeks (P <.01). Pingley et al.142 also noted a correlation between treatment time and outcome; patients receiving brachytherapy within 4 weeks of external-beam radiation therapy (EBRT) had a 5-year disease-free survival rate of 60%, compared with a 30% rate in patients who had an interval >4 weeks.
Treatment: Surgery
For most patients with invasive vaginal cancer, radiation is the treatment of choice. Surgery is considered for highly selected patients who have early-stage lesions, when a potentially curative resection can be achieved without extensive functional morbidity. Surgery is also used for previously irradiated patients who cannot receive further radiation. A wide local excision is reserved only for carcinoma in situ or small, superficially invasive lesions that are well demarcated. More extensive lesions in the proximal aspect of the vaginal canal require radical hysterectomy, upper vaginectomy, and bilateral pelvic lymphadenectomy, and patients with positive margins require adjuvant radiation. Lesions that extend to the inferior vagina require a total vaginectomy with radical hysterectomy, pelvic lymphadenectomy, and possibly vulvovaginectomy and inguinofemoral lymphadenectomy.89,90,98,99 It is not uncommon for relatively small lesions to invade the rectum or urethra early in the disease course, given the close proximity of the vagina to these structures. Older surgical series often required pelvic exenteration in 40% to 50% of cases to obtain negative margins.95,99 Anterior exenteration removes the vagina, urethra, and bladder and is often necessary to achieve negative margins for invasive anterior wall lesions. Posterior exenteration requires resection of the vagina and rectum. Deeply invasive, circumferential lesions may require a total exenteration in order to achieve clear margins. Given the potentially devastating functional results associated with radical surgery, definitive radiation is the treatment of choice for most patients with invasive vaginal cancer and has largely replaced surgery as the primary therapeutic modality.
In select stage I patients, surgery can offer excellent results, with series reporting 5-year survival rates ranging from 56% to 100% for women with stage I disease.4,89,95,98,132,144 The NCDB review for cancers of the vagina noted superior survival rates in patients treated with surgery,4 although this likely reflects selection of healthier patients with good performance status for radical surgery. A more recent analysis utilizing the SEER database1 found that women with stage I disease who underwent surgery only, had a lower risk of mortality than those treated with radiation only, combined modalities, or no treatment; however, this difference did not reach statistical significance. For stage II vaginal cancer patients, there was a similar trend toward increased mortality in women who did not have surgery alone as their primary treatment modality, but values once again did not reach statistical significance in their multivariate adjusted model.
In a review of 100 cases by Stock et al.98 surgical treatment was noted to be a significantly favorable prognostic factor for disease-free survival, versus treatment with radiation alone, in stage II patients but not stage I patients. For stage I patients, survival rates were 56% and 80% for patients treated with surgery versus radiation, respectively. For stage II patients, survival rates were 68% and 31% after surgery and radiation, respectively, although this likely reflects selection bias, with patients with more extensive involvement offered radiation. Overall 5-year survival was 47%. Stock et al.98 concluded that surgery that consists of radical hysterectomy, pelvic lymphadenectomy, and upper vaginectomy could be reasonable for stage I lesions and select stage II lesions, with radiation being the preferred primary modality for patients with stage IIB disease. It should be noted, however, that 23 of 33 stage II patients (70%) treated with surgery required a total vaginectomy or exenterative procedure, which carries significant morbidity and functional impairment.
Other series also report excellent results with primary surgical therapy, although authors acknowledge bias resulting from selection of healthier patients with less extensive disease for primary surgery over radiation. Tjalma et al.132 reported on 55 cases of primary vaginal SCC. Of 27 patients with stage I disease, 26 received surgery, with 4 subsequently receiving some form of adjuvant radiation. With a median follow-up time of 45 month, 5-year survival was reported to be 91%. Otton et al.,144 in their retrospective review of 70 patients with stage I or II vaginal carcinoma treated at Queensland Centre for Gynaecological Cancer between 1982 and 1998, report that patients treated with surgery alone, or a combination of surgery and radiation, had significantly longer survival times than patients treated with radiation alone. The authors suggest that surgery may be effective in a select subset of patients with small, localized tumors that permit clear surgical margins. Peters et al.106 reviewed records of 86 patients with vaginal carcinoma, including 68 SCC cases, treated at University of Michigan Medical Center. Twelve selected patients had surgery as primary therapy, with a 75% survival rate. Similarly, Rubin et al.99 reported on eight patients with stage I or II disease who received surgery as primary treatment; 5-year survival was 75%, and the overall local control rate for the stage I patients was 80%, suggesting that highly selected patients can achieve excellent outcomes with surgery. Davis et al.95 reported on 89 patients with vaginal carcinoma treated primarily at the Mayo Clinic from 1960 to 1987. A total of 52 patients were treated with surgery as primary therapy, with 5-year survival of 85% compared with 65% for patients who received radiation alone. In the stage II patients, the 5-year survival rates were 49%, 50%, and 69% for surgery, radiation, and combined treatment with surgery and radiation, respectively. However, treatment modalities cannot be effectively compared using retrospective series, which reflect strong selection biases.
Ling et al.,145 in a small series with 4 patients who had stage I disease, report their experience using laparoscopic radical hysterectomy with vaginectomy and reconstruction of the vagina. With follow-up times ranging from 40 to 54 months, they reported all patients to be free of disease, with satisfactory sexual function. The authors suggest that laparoscopic surgery can be an option for select patients with early-stage disease, with good outcomes.
Several series report their experience using surgery for advanced stage III or IV patients, with most cases requiring pelvic exenteration.89,90,98,99 Control rates at best were 50% in highly selected patients. In practice, given the overall poor prognosis and morbidity associated with surgery, advanced-stage patients should receive treatment with definitive radiation, typically in combination with chemotherapy.
Neoadjuvant chemotherapy followed by radical surgery has been proposed for selected patients with vaginal cancer.146,147 Benedetti et al.147 reported results on 11 patients with stage II SCC of the vagina, using 3 cycles of neoadjuvant paclitaxel and cisplatin. Ninety-one percent of patients obtained a partial or complete response to neoadjuvant chemotherapy; 27% achieved a complete response. All patients had disease-free resection margins after surgery, and only one patient had positive lymph nodes. At a median follow-up time of 75 months, 10 of 11 patients (91%) were alive, and of those, 8 (73%) were free of disease. Postoperative complications were mild. A case report documented the use of neoadjuvant chemotherapy, consisting of bleomycin and cisplatin, followed by radical surgery in one patient with stage II SCC of the vagina.146 The patient was free of disease, with satisfactory sexual function, at 30 months. However, larger series of patients treated with this approach, with longer follow-up, are necessary to further evaluate the feasibility of this treatment.
Treatment: Radiation
Stage I
It is difficult to compare results for stage I and stage II disease from different series, as the distinction between them is made clinically, based on physical examination, and can be subject to variability. In general, stage I lesions are 0.5 to 1 cm in thickness. It is important to individualize radiation therapy techniques based on size, depth, and location of the lesion.
Selected patients with small, superficial tumors may be adequately treated with brachytherapy alone, with reported local control rates of 67% to 100%.92,93,97,103,114,135,148,149 Perez et al.63 reported pelvic tumor control of 88% in patients with stage I disease who received brachytherapy alone, using a dose of 60 to 70 Gy, prescribed 5 mm beyond the plane of the implant or vaginal mucosa, with a vaginal surface dose of 80 to 120 Gy. Frank et al.114 reported on 21 patients with stage I disease who were treated with local radiation only, without regional node coverage. Nine received brachytherapy alone, 11 received EBRT with or without brachytherapy, and 1 received local EBRT using a transvaginal orthovoltage cone. Three of 9 patients treated with brachytherapy alone developed recurrent disease in the pelvis, resulting in a 10-year pelvic disease control rate of 67%. Patients who had received EBRT with or without brachytherapy did not have pelvic recurrences. In the series by Dancuart et al.,148 patients treated with brachytherapy or transvaginal cone irradiation alone had a local failure rate of 18%. A pelvic relapse rate of 18% at 10 years was noted by Frank et al.,114 with all pelvic failures occurring in patients treated with brachytherapy alone.
Typically, the entire length of the vagina is treated to a mucosal dose of 60 to 65 Gy, with an additional mucosal dose of 20 to 30 Gy delivered to the area of tumor involvement.150 With LDR, treatment can be delivered in two applications, with the first designed to treat the entire vaginal wall and a second application to cover the tumor volume. This can be delivered with a shielded vaginal cylinder to treat the tumor with a 2-cm margin and block uninvolved mucosal surfaces. HDR can also be used to treat superficial lesions. In general, the vaginal mucosa is treated to a dose of 21 to 25 Gy, prescribed to a depth of 5 mm, in weekly fractions of 5 to 7 Gy each. An additional 21 to 25 Gy, prescribed to a depth of 5 mm, is delivered to the tumor via shielded vaginal cylinder, with weekly fractions of 5 to 7 Gy, to bring the total dose to 42 to 50 Gy. For lesions thicker than 5 mm, a combination of intracavitary and interstitial brachytherapy can be utilized. For such lesions, a vaginal cylinder typically delivers 45 Gy (LDR) or 21 to 25 Gy (HDR) to a depth of 5 mm into the vaginal mucosa. Subsequent therapy is delivered via interstitial implant, to deliver an additional dose of 25 to 35 Gy (LDR) to the tumor volume.
A combination of EBRT and brachytherapy is suggested for more extensive stage I lesions that exhibit greater infiltration or poor differentiation. Perez et al.63 noted that tumor control in stage I vaginal carcinoma was approximately the same with brachytherapy alone as when given in combination with EBRT, consistent with observations made by some groups137,151 but not others.114 Given possible underestimation of submucosal disease or nodal disease, resulting in a potentially high likelihood of recurrence with brachytherapy alone, some groups recommend incorporating EBRT into treatment of all stage I patients, except for those with very small, superficial lesions.114 Frank et al.,114 in their series of patients with vaginal cancer treated at MDACC between 1970 and 2000, noted an increased trend toward increasing use of EBRT for stage I vaginal SCC over time.
Actuarial 5-year survival rates for stage I disease range from 60% to 85%.1,93,114,131 Disease-specific survival rates for stage I disease, treated with definitive radiation, range from 75% to 95%.63,60,94 The 10-year pelvic-relapse rate, comprising local, pelvic nodal, and inguinal nodal failures, was noted to be 16% by Frank et al.114 for stage I patients. Distant metastases are uncommon and occur in about 5% of patients.63,95,148
Stage II
Radiation is the primary treatment for stage II disease and involves a combination of EBRT and brachytherapy. Perez et al.63 noted a 36% pelvic tumor control rate in stage II patients treated with brachytherapy alone, compared with 67% in patients treated with a combination of EBRT and brachytherapy. The benefit of combining EBRT and brachytherapy, as opposed to using either alone, has been shown in other series as well.60,98
Generally, patients with stage II disease are treated with EBRT followed by interstitial or intracavitary brachytherapy. The pelvis receives 45 to 50.4 Gy in 1.8 Gy fractions, with consideration of a parametrial boost if there is extensive primary infiltration or high suspicion of nodal disease. Inguinal lymph nodes are included in a modified whole pelvic field for lesions involving the distal vaginal canal.
Chyle et al.60 reported an 89% local-control rate in the vagina for patients treated with brachytherapy alone, although the rate of pelvic wall relapse was not reported in this cohort. Of 28 patients treated with EBRT alone, with carefully designed shrinking fields, three (11%) developed vaginal recurrences. In comparison, there were 12 recurrences (21%) in 58 patients treated with combined EBRT and brachytherapy. The authors concluded that coverage of the entire tumor volume is critical for optimal outcome.
Brachytherapy should be carefully delivered to ensure adequate coverage of tumor volume. An interstitial technique, ideally with three-dimensional (3D) imaging for treatment planning, is required for tumors >5 mm in depth.92,152 Extensive tumors, or deeply infiltrating tumors with nondistinct margins, may be poor candidates for brachytherapy. In such cases, boosting tumors with conformal techniques or intensity-modulated radiation therapy (IMRT) may be preferred and may yield better outcomes than suboptimal brachytherapy.114 The tumor volume should receive a minimum of 75 to 80 Gy using combined EBRT and brachytherapy. Fleming et al.153 and Puthawala et al.154 both report improved outcomes with higher doses of 80 to 100 Gy.
The 5-year survival rate for patients with stage II disease treated with radiation therapy alone ranges from 35% to 70% for stage IIA to 35% to 60% for stage IIB.29,153 Pelvic relapse at 10 years has been reported to be 25% by Frank et al.,114 consistent with recent series reporting 5-year pelvic-control rates ranging from 76% to 84%.131 The likelihood of distant metastasis is higher for stage IIB lesions compared with stage IIA,93,151 with overall reported rates ranging from 22% to 46%.93,95
Stages III and IVA
Patients with more advanced disease generally also receive EBRT to the pelvis, followed in certain cases by additional dose to the parametrium. If adequate tumor coverage can be achieved without undue toxicity, interstitial brachytherapy is employed to deliver a minimum tumor dose of 75 to 80 Gy. If brachytherapy is not feasible, due to extensive tumor infiltration of the rectovaginal septum or bladder, a shrinking-field technique or IMRT has been used to deliver additional dose to the primary lesion.155,156 The overall cure rate for patients with stage III disease ranges from 30% to 50%. Stage IVA carries a worse prognosis. In highly selected patients with small volume stage IV disease, pelvic exenteration can yield good long-term control; however, in practice, EBRT remains the primary treatment.1,4,63,98,99,114,135,157,158 Five-year actuarial survival rates for women with stage III disease range from 25% to 58%,1,4,159 with local failure rates of 30% to 75%.93,114,131 Outcomes for stage IV disease are worse, with survival rates of 0% to 40%.60,98,160 Despite treatment with EBRT and brachytherapy, only 20% to 30% of patients with stages III and IV disease achieve local control. Pelvic recurrences occur more often than distant recurrences.114
Role of Chemotherapy and Radiation
There are no randomized trials that compare radiation alone with radiation plus chemotherapy in vaginal cancer, and many studies of chemoradiation for primary vaginal cancer are limited by small numbers or inclusion of other cancers, such as cervical and vulvar carcinomas. However, many clinicians incorporate the use of cisplatin for treatment of vaginal cancers, extrapolating from data demonstrating improved progression-free and overall survival in cervical cancer when cisplatin is added to radiation.63,161–164
Holleboom et al.165 published a case report documenting the use of cisplatin with EBRT and brachytherapy in a patient with advanced stage SCC of the vagina. The patient was free of disease at 16 months. Evans et al.166 reported the use of radiation with 5-FU and mitomycin-C (MMC) in seven patients with vaginal cancer. Four of seven patients were free of disease with follow-up times ranging from 19 to 39 months. Roberts et al.167 reported results for seven patients with vaginal cancer treated with concurrent 5-FU, cisplatin, and radiation. Three patients received interstitial brachytherapy after EBRT, and two patients received intracavitary brachytherapy after EBRT. Eighty-five percent of patients achieved a complete response initially. Ultimately, 61% recurred, with a median time to recurrence of 6 months. There were three local recurrences and one distant metastasis and the 5-year overall survival rate was 22%. Kirkbride et al.91 reported on the use of concurrent 5-FU, with or without MMC, in 26 of 153 patients with vaginal carcinoma treated at Princess Margaret Hospital. Seventy-seven percent of the patients had stage III or IV disease. Radiation was EBRT followed by interstitial or intracavitary brachytherapy to a total dose of 62 to 74 Gy. The 5-year survival rate was 50%. Dalrymple et al.168 reported results using 5-FU-based chemotherapy in combination with radiation for treatment of primary SCC of the vagina. Thirteen of 14 patients (93%) had stage I or II disease. The median dose of radiation was 63 Gy, achieved using EBRT alone or EBRT with intracavitary brachytherapy. The 5-year survival rate was 86% for all patients, and nine patients were free of disease with a median follow-up time of 100 months, suggesting that good local control can be achieved despite the use of lower radiation doses. There was a 31% rate of severe bowel complications reported, with two deaths as a result of bowel obstruction.
A retrospective series from MDACC by Frank et al.114 included nine patients with stage II or IVA SCC of the vagina treated with radiation therapy and concurrent cisplatin-based chemoradiation. With a mean follow-up time of 129 months, improved local control with the use of chemotherapy was noted, with 44% of patients treated with concurrent chemoradiation remaining free of disease. Samant et al.169 published a review of 12 vaginal cancer patients, stage II to IVA, treated with concurrent weekly cisplatin at a dose of 40 mg/m2 for 5 weeks. Patients received concurrent EBRT to a median dose of 45 Gy, with LDR interstitial or an HDR intracavitary brachytherapy boost of median dose 30 Gy. Six patients had stage II disease, four had stage III disease, and two had stage IVA. Ten of 12 (83%) patients had SCC; the other 2 had adenocarcinoma. Overall, treatment was well tolerated, with 92% of patients completing therapy as prescribed. Two of 10 patients who received interstitial brachytherapy required surgery for fistula repair. The 5-year overall survival, progression-free survival, and locoregional progression-free survival rates were 66%, 75%, and 92%, respectively, supporting use of concurrent weekly cisplatin therapy. A small series of six patients treated with chemoradiation at the University of the Ryukyus was reported by Nashiro et al.170 All patients received EBRT to 50 Gy, followed by either a boost with shrinking fields (n = 4) or intracavitary brachytherapy (n = 2). Radiation was delivered with two to three cycles of cisplatin. Two patients had stage II, one had stage III, and three had stage IVA disease. All six achieved a complete response, and four of six patients remained free of disease at follow-up times of 18 to 55 months.
In a retrospective analysis of 71 patients with primary vaginal cancer treated at Dana-Farber Cancer Institute/Brigham and Women’s Hospital from 1972 to 2009, 51 patients were treated with radiation alone and 20 were treated with chemotherapy and radiation.170 Of patients treated with chemosensitization during radiation, 85% of patients received weekly cisplatin chemotherapy, while the remainder received either carboplatin or 5-FU. Three-year actuarial overall survival and disease-free survival was 56% for the radiation alone group, compared with 79% for the chemoradiation group (P = .01). Three-year disease-free survival was 43% for the radiation alone group, compared with 73% for the chemoradiation group (P = .01). At a median follow-up of 3 years, tumor relapse was seen in 15% of patients treated with chemoradiation compared with 45% of patients treated with radiation alone (P = .03).
Ghia et al.172 published a retrospective patterns-of-care analysis using the SEER database, analyzing data from women with primary vaginal cancer treated with EBRT or brachytherapy between 1991 and 2005. Of the 326 women in the study cohort, 80.4% had SCC. It was noted that chemoradiation was used in 7.5% of patients treated before 1999 compared with 36.1% of those treated afterward (P <.001). Cisplatin was the most frequently utilized agent, accounting for 59% of chemoradiation treatments. Chemotherapy was significantly less likely to be used in conjunction with radiation for women over 80 years of age; otherwise, there was no difference for race, stage, grade, histologic diagnosis, comorbidities, or brachytherapy use. On multivariate analysis, chemoradiation was not found to correlate with improved cause-specific or overall survival.
TABLE 72.4 OUTCOMES FOR VAGINAL CANCER BY TREATMENT MODALITY