RENAL CANCER
I. EPIDEMIOLOGY AND ETIOLOGY
A. Incidence. Renal cell carcinoma (RCC) constitutes 3% of adult malignancies. The worldwide incidence is increasing at an annual rate of about 2%, with approximately 58,000 new cases per year in the United States and 13,000 associated deaths. Men are affected twice as often as women. RCC is a tumor of adults, occurring primarily in those in their fourth and sixth decades. The incidence and mortality rates for blacks appear to be increasing in excess to those for whites in the United States.
B. Etiology. Approximately 70% of sporadic cases of clear cell RCC (the most common histologic variant) are associated with inactivating mutations of both copies of the Von Hippel-Lindau (VHL) tumor suppressor gene. This results in overexpression of hypoxia inducible factor-1 (HIF-1) and vascular endothelial growth factor (VEGF), leading to defective regulation of angiogenesis, which is of major importance in the pathophysiology of RCC.
1. Factors that increase the risk for RCC include the following:
a. Smoking
b. Urban living
c. Family history of renal cancer
d. Thorotrast exposure
e. Obesity
f. Genetic syndromes include
(1) Von Hippel—Lindau disease (associated with germline mutations of the VHL gene; 35% to 45% of these patients have RCC, mostly multiple and bilateral)
(2) Hereditary type 2 papillary RCC: associated with mutations in MET proto-oncogene
(3) Birt-Hogg-Dube (BHD) syndrome
2. Unproven factors that may increase the risk for RCC include polycystic kidney disease, diabetes mellitus, and chronic dialysis.
II. PATHOLOGY AND NATURAL HISTORY
A. Adenocarcinomas (historically named hypernephromas or Grawitz tumors) make up nearly all renal cancers in adults. They are typically round and have a pseudocapsule of condensed parenchyma and connective tissue. Bilateral tumors occur in 2% of sporadic cases, either synchronous or asynchronous.
1. The most common histologic types include clear cell (70%), papillary (10% to 15%), chromophobe (5%), and unclassified RCC (<5%). Sarcomatoid tumors can arise from any cell subtype.
2. These tumors originate from proximal tubular cells, invade local structures, and frequently extend into the renal vein. Metastasis occurs through the lymphatics and bloodstream. The most common sites of distant metastases are the lungs, liver, bones, and brain. Adenocarcinomas may present with metastases to
unusual sites, such as the fingertips, eyelids, and nose. A primary renal cancer may be diagnosed based on the characteristic histology of a metastatic deposit.
3. The natural history of RCC is more unpredictable than that of most solid tumors. The primary tumor has variable growth patterns and may remain localized for many years. Metastatic foci may have long periods of indolent or apparently arrested growth and may be detected many years after removal of the primary tumor.
B. Transitional cell carcinomas are uncommon tumors that arise in the renal pelvis and often affect multiple sites of urothelial mucosa, including the renal pelvis, ureters, and urinary bladder (see “
Urinary Bladder Cancer,” Section II). These tumors usually are low grade but are being discovered late in the course of the disease. Transitional cell carcinomas occasionally have a peculiar disposition to spread over the posterior retroperitoneum in a sheet-like fashion, encasing vessels and producing urinary tract obstruction. Hematogenous dissemination occurs, particularly to lung and bone.
C. Rare renal tumors
1. Nephroblastomas (Wilms tumors) appear as large, bulky masses in children but rarely occur in adults (see
Chapter 18, “Wilms Tumor”).
2. Lymphomas and sarcomas arising in the kidney have clinical courses similar to their counterparts elsewhere in the abdomen.
3. Juxtaglomerular tumors (reninomas) are rare causes of hypertension and are usually benign.
4. Hemangiopericytomas are renin-secreting tumors associated with severe hypertension and are occasionally malignant (15% of cases).
5. Oncocytomas (7%) are benign tumors originating from a subtype of collecting ducts.
6. Bellini tumors (collecting duct RCC, <1%) are aggressive cancers originating from collecting ducts.
7. Medullary cancer (<1%)
8. Benign renal adenomas. The existence of benign renal adenoma is controversial because it is not possible to determine malignant or benign biologic behavior only by histology on any lesion <3 cm in diameter.
D. Metastatic tumors. The kidney is a frequent metastatic landing site for many malignancies, mainly cancers of the lung, ovary, colon, and breast.
E. Paraneoplastic syndromes commonly occur with renal adenocarcinomas.
1. Erythrocytosis. Renal adenocarcinomas are associated with erythrocytosis in 3% of patients and account for 15% to 20% of cases of inappropriate secretion of erythropoietin. A left flank mass of RCC may be mistaken for an enlarged spleen resulting from polycythemia vera. The differential diagnosis of erythrocytosis is discussed in
Chapter 34, “Increased Blood Cell Counts,” Section I. Tumor production of erythropoietin may identify a subset of patients responsive to immunotherapy with interleukin-2 (IL-2) and interferon-α (IFN-α).
2. Hypercalcemia, which occurs in about 5% of patients, associated with parathyroid hormone-like proteins. Hypercalcemia may also be associated with widespread bony metastases.
3. Fever caused by tumor occurs in 10% to 20% of patients.
4. Abnormal liver function (Stauffer syndrome) occurs in 15% of patients. Leukopenia, fever, and areas of hepatic necrosis without liver metastases are noted. The resulting elevated serum levels of alkaline phosphatase and transaminase are reversed after nephrectomy.
5. Hypertension associated with renin production by the tumor occurs in up to 40% of patients and is alleviated by removal of the tumor.
6. Hyperglobulinemia can result in elevated erythrocyte sedimentation rate.
7. Amyloidosis occasionally occurs.
IV. STAGING SYSTEM AND PROGNOSTIC FACTORS
A. Staging system. The current TNM staging system is shown in
Table 13.1. A different system is used for cancers of the renal pelvis.
B. Prognostic factors
1. Pathologic stage is the most important prognostic indicator.
a. Tumor size >10 cm is associated with poor prognosis in comparison to smaller lesions.
b. Venous extension. Renal vein or vena caval involvement is not associated with a hopeless prognosis if managed properly; 25% to 50% of patients survive for 5 years.
2. Histology. Sarcomatoid and unclassified patterns of RCC have a poor prognosis.
a. Nuclear grade correlates with survival across all tumor stages. Fuhrman four-tiered system is most commonly used; it takes into consideration nuclear size, nuclear shape, and nucleolar appearance.
b. Nuclear ploidy was proposed as a potential prognostic marker for survival. Nondiploid tumors are thought to harbor a less favorable prognosis.
3. Disease-free interval. The length of time between nephrectomy and the development of metastases affects the survival of patients with metastatic disease.
a. Nearly all patients who have metastases at the time of surgery or who develop metastases or local recurrence within 1 year of surgery die within 2 years if untreated.
b. Patients who develop metastases >2 years after nephrectomy have a 20% 5-year survival rate from the time metastases are recognized.
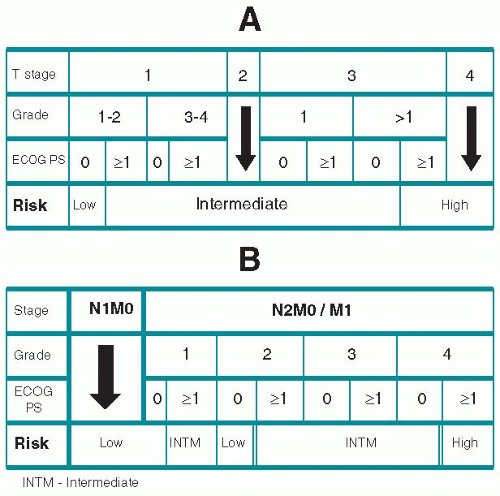
4. Integrated prognostic systems. The TNM system can be augmented with more complex systems that take into account various prognostic factors, such as Fuhrman nuclear-grading system and performance status to assess risk and the probability of survival with and without evidence of RCC. Such a system is shown in
Figure 13.1.
V. PREVENTION AND EARLY DETECTION
The incidence of renal cancer might be reduced if tobacco-smoking habits could be controlled. Early detection depends on prompt attention to hematuria and other symptoms suggestive of these cancers.
VI. MANAGEMENT
A. Early disease
1. Surgery
a. Radical nephrectomy classically involves removal of all structures contained within Gerota fascia, including kidney, adrenal gland and superior ureter. Radical nephrectomy is generally used for large, locally advanced tumors. The adrenal gland can be safely spared when the tumor is in the lower pole or when a smaller tumor is clearly separate from the adrenal gland. A laparoscopic approach is generally preferred; however, an open approach may be necessary for tumors that involve adjacent structures such as the inferior vena cava.
b. Nephron-sparing surgery (NSS, partial nephrectomy) is the treatment of choice when it is technically feasible. Maximal preservation of renal function is an important goal of surgery since even mild renal insufficiency has been linked to cardiovascular disease and related morbidities such as myocardial infarction and stroke. Most tumors that are <4 cm in diameter are amenable to NSS. A minimally invasive approach using laparoscopy or robotics results in more rapid postoperative recovery when compared to an open approach. Although surgical resection is considered the standard of care, ablative procedures using cryotherapy or radiofrequency ablation can be considered for the smallest and most exophytic tumors.
c. Occlusion of the renal artery using angiographic techniques has been advocated for locally advanced tumors associated with increased vasculature. Occlusion procedures may limit blood loss and make the operation
technically easier. It may also provide palliation for symptomatic patients who are not candidates for surgery. However, renal artery occlusion will cause temporary pain, fever, and nausea.
d. Contraindications to surgery include high surgical risk because of unrelated medical diseases. Since the emergence of targeted therapies, the role of surgery (“adjunctive nephrectomy”) in the presence of distant metastases is once again under investigation.
2. Observation is now recognized as an acceptable option for small renal tumors (e.g., tumor <4 cm) in patients who are poor surgical candidates or have a limited life-expectancy. Many of these small tumors are benign; however, even if they are malignant, most small tumors are indolent and progress slowly.
3. RT has no established role in the management of early renal cancers.
4. Chemotherapy has no established role in the management of early renal cancers.
B. Advanced disease
1. Surgery
a. Nephrectomy. For patients treated with immunotherapy, cytoreductive nephrectomy has been shown to extend survival. However, immunotherapy has been largely replaced by a growing number of targeted therapies such as sunitinib and sorafenib. Therefore, ongoing clinical trials are revisiting the role of cytoreductive nephrectomy in patients treated with targeted therapies. Until these trials are completed, cytoreductive nephrectomy remains a well-accepted adjuvant to systemic therapy for patients with good performance status.
b. Resection of metastases. In select patients, metastatic lesions can be surgically resected for curative intent. Metastectomy is most likely to be curative in patients with a single metastatic lesion and in patients with a solitary recurrence identified more than 2 years after the definitive nephrectomy. Prior to performing metastectomy, a thorough metastatic workup is mandatory.
2. RT is used to palliate symptoms from metastases to the central nervous system and bone. Gamma knife radiotherapy is effective for control of brain metastasis.
3. Pharmacotherapy
a. Targeted Agents: Six molecularly targeted therapies are currently approved for the treatment of advanced RCC. These can be broadly divided into two categories: (1) vascular endothelial growth factor (VEGF)-directed therapies and (2) inhibitors of the mammalian target of rapamycin (mTOR). VEGF-directed agents include the monoclonal antibody bevacizumab, and the small molecule tyrosine kinase inhibitors (TKIs) sunitinib, sorafenib, and pazopanib. mTOR inhibitors currently approved for RCC therapy include everolimus and temsirolimus.
Each of these agents is supported by phase III data which can guide their clinical utilization. For instance, sunitinib and bevacizumab (with interferon-α, IFN-?α) were evaluated in treatment-naïve patients with advanced clear cell RCC. In contrast, the pivotal trial of pazopanib was performed in patients who were either treatment-naïve or cytokine-refractory. The majority of patients (82%) treated in the phase III evaluation of sorafenib were also cytokine-refractory. Given these reports, the National Comprehensive Cancer Network (NCCN) has rendered a category 1 recommendation to sunitinib, pazopanib, and bevacizumab/IFN-?α, for the first-line therapy of advanced clear cell RCC. Pazopanib also carries a category 1 recommendation for patients with cytokine-refractory disease, as does sorafenib. A category 1 recommendation by NCCN is based on a high level of evidence (e.g., randomized controlled trials) with uniform NCCN consensus.
The pivotal trials for everolimus and temsirolimus assessed distinct populations. Patients in the phase III evaluation of everolimus had advanced clear cell RCC and had been exposed to previous treatment with sunitinib and/or sorafenib. In contrast, the pivotal trial of temsirolimus principally included treatment-naïve patients with poor-risk disease. Uniquely, eligibility was not limited to those patients with clear cell RCC, and patients with treated brain metastases were allowed to enroll. The agent now carries a category 1 recommendation from the NCCN for patients with poor-risk disease.
More extensive experience with VEGF-directed therapies and mTOR inhibitors has suggested class effects associated with these agents. For
instance, VEGF-directed therapies appear to cause hypertension, proteinuria, hand-foot syndrome, impaired wound healing, and myelosuppression. In contrast, mTOR inhibitors have been associated with impaired metabolic profiles (i.e., hyperglycemia, hypertriglyceridemia, etc.), mucositis, and rash.
b. Immunotherapy. The role of immunotherapy for kidney cancer has decreased and is being supplanted by rapid progress with antiangiogenic agents. Nonetheless, IL-2 remains the only potentially curative treatment in selected patients with metastatic RCC.
(1) IL-2 administered alone in high-dose regimens produces a response rate of 15% to 20% in good-risk patients and durable remissions lasting for more than a decade in 10% of patients. Significant morbidity and 4% mortality associated with high-dose IL-2 make this therapy very difficult and applicable to only small minority of patients. IL-2 administered in lower dosages or in combination with IFN produces inferior response rates when compared with high-dose IL-2 in randomized trials. Recent data from the Renal SELECT study underscore a dearth of clinical or biologic criteria that identify patients who yield benefit from this modality.
(2) IFN-α as a single agent has modest antitumor activity in the setting of RCC, with a response rate of approximately 15%. With the emergence of effective targeted therapies, IFN-α is used primarily in clinical trials evaluating possible synergistic effects in combination with antiangiogenic agents.
c. Future strategies in the treatment of RCC include redefining the role of nephrectomy in advanced cases in the era of targeted therapies, the development of novel immunotherapeutic strategies (i.e., vaccine based therapy or PD-1 inhibition), and the development of biologic tools to risk stratify patients with both localized and advanced disease.
URINARY BLADDER CANCER
I. EPIDEMIOLOGY AND ETIOLOGY
A. Incidence. Bladder cancers constitute 4.5% of all cancers in the United States. The disease is 2.5 times more frequent in men than in women and is most frequent in industrial northeastern cities. The average age of onset is the sixth to seventh decade. The incidence doubles in men >75 years of age versus younger men.
B. Risk factors and carcinogens
1. Occupational exposure is associated with 20% of cases. Historically, aniline dye workers were afflicted 30 times more than the general population. Aromatic amines and related compounds are the most abundant bladder carcinogens today. These are chemical intermediates of anilines, rather than the aniline dyes themselves. Leather, paint, and rubber industry workers also appear to have an increased risk for bladder cancer. Proven chemical carcinogens in these industries are 2-naphthylamine, benzidine, 4-aminobiphenyl, and 4-nitrobiphenyl.
2. Schistosomum haematobium infection of the bladder is associated with bladder cancer, particularly with squamous cell histology, in endemic regions of Africa and the Middle East.
3. Smoking increases the risk for bladder cancer fourfold in a dose-dependent fashion. Of men who die of bladder cancer, 85% have a history of smoking.
4. Pelvic irradiation increases the risk for bladder cancer fourfold.
5. Drugs. Cyclophosphamide unequivocally increases the risk for bladder cancer. Other drugs that have been implicated in animal studies but not proved in humans are phenacetin, sodium saccharin, and sodium cyclamate.
II. PATHOLOGY AND NATURAL HISTORY
A. Pathology
1. Histology. Of bladder cancers, 90% are transitional cell carcinoma (TCC), and 8% are squamous cell types. Adenocarcinomas, sarcomas, lymphomas, and carcinoid tumors are rare.
2. Sites of involvement. The majority of TCCs are linked to carcinogen exposures such as smoking. Carcinogens in urine are believed to produce a fieldchange in the urothelium that predisposes to formation of bladder cancer. Therefore, TCC can develop in any part of the urinary collecting system including the kidney and ureter; however, the bladder is the most common site for TCC because it functions to store urine and has the greatest contact time with urinary carcinogens.
3. Types of bladder cancer
a. Single papillary cancers are the most common type (70%) and the least likely to show infiltration.
b. Diffuse papillary growths with minimal invasion
c. Sessile cancers are often high grade and invasive.
d. Carcinoma in situ (CIS; flat intraepithelial growth) appears either the same as normal mucosa or as a velvety red patch.
4. The panurothelial abnormality or field defect. Bladder cancer appears to be associated with premalignant changes throughout the urothelial mucosa. This concept is suggested by the following observations:
a. Up to 80% of patients treated for superficial tumors develop recurrences at different sites in the bladder.
b. Multiple primary sites are present in 25% of all patients with bladder cancer.
c. Random biopsies of apparently normal areas of mucosa in patients with bladder cancer frequently show CIS.
d. Depending on the reported series, patients with bladder CIS also have ureteral CIS (10% to 60%) and urethral CIS (30%).
e. About 40% of patients presenting with carcinoma of the renal pelvis or ureter develop tumors elsewhere in the urinary tract, usually in the bladder.
B. Natural history
1. CIS of the bladder is multifocal and can affect the entire urothelium. CIS is a high-grade malignancy. Up to 80% of patients with untreated CIS develop invasive bladder cancer within 10 years after diagnosis; the disease is lethal for most of these patients.
2. Low-grade superficial carcinomas have a better prognosis than CIS. Although the recurrence rate is 80%, low-grade, superficial carcinomas do not metastasize. However, 10% of superficial carcinomas may progress to high grade, invasive tumors with potential for metastasis. More than 80% of patients with both superficial cancers and CIS progress to invasive disease.
3. High-grade or invasive tumors are associated with adjacent areas of CIS in 85% of cases. Squamous cell cancers and adenocarcinomas are usually high grade and have an aggressive clinical behavior. Other uncommon and very aggressive histologic variants include sarcomatoid cancer, small cell carcinoma, and micropapillary tumors.
4. Mode of spread. Bladder cancers spread both by lymphatic channels and by the bloodstream. High-grade lesions are more likely to metastasize. Of patients with distant metastases, 30% do not have involvement of the draining lymph nodes. Distant sites of metastases include bone, liver, lung, and, less commonly, skin and other organs. Uremia from ureteral compression by a large pelvic mass, inanition from advancing cancer, and liver failure are the usual causes of death.
5. Iatrogenic tumor implantation. High-grade bladder cancer cells exfoliated by cystoscopy, brushing, transurethral biopsy, or resection were reported to seed other areas of the bladder. Mucosal sites damaged by inflammation or instrumentation appear to be most receptive to such implants.
6. Associated paraneoplastic syndromes
a. Systemic fibrinolysis
b. Hypercalcemia
c. Neuromuscular syndromes
d. Leukemoid reaction
IV. STAGING SYSTEM AND PROGNOSTIC FACTORS
A. Staging system. The current TNM staging system is shown in
Table 13.2.