I. GENERAL ASPECTS OF HEAD AND NECK CANCERS.
Head and neck cancers include a heterogeneous group of malignant tumors arising in all structures cephalad to the clavicles, except for the brain, spinal cord, base of the skull, and usually the skin. A meaningful understanding of these malignant tumors requires anatomic separation into those cancers arising in the oral cavity, oropharynx, hypopharynx, nasopharynx, larynx, nasal fossa, paranasal sinuses, thyroid and salivary glands, and vermilion surfaces.
A. Epidemiology and etiology
1. Incidence. The incidence of head and neck cancer continues to increase worldwide. Cancers arising in the head and neck constitute about 3% of all newly diagnosed cancers in humans. Head and neck cancer is the 10th most common cancer worldwide, with more than 45,000 new cases reported every year in the United States alone.
2. Etiology
a. Cigarette smoking and alcohol intake are the major risk factors. The use of tobacco products (including cigarettes, cigars, pipes, chewing tobacco, and snuff) and substantial alcohol intake are the major risk factors for head and neck carcinoma. Drinking alcohol and using tobacco at the same time more than doubles the risk of developing head and neck cancer.
Carcinogens in these products can induce molecular changes throughout the entire upper aerodigestive tract. Slaughter first described these changes, originating the concept of field carcinogenesis or “condemned mucosa” in 1953. Slaughter hypothesized that because of constant carcinogenic pressure, the entire upper aerodigestive tract is at increased risk of developing tumors. Head and neck cancer results from a multistep carcinogenesis process in which increasing degrees of mucosal changes and cellular atypia occur over large areas of the carcinogen-exposed upper aerodigestive tract epithelium. Of the survivors of one cancer of the head and neck, 20% develop another primary head and neck cancer.
b. Human papillomavirus (HPV) is now recognized as a risk factor for oropharyngeal cancer, independent of tobacco or alcohol usage. HPV-positive cancer cases are now in majority in the Western world, and these tumors are also shown to have better outcome than HPV-negative patients (discussed further in Section IV. Oropharynx.C.1).
B. Pathology
1. Histology. Nearly all cancers of the oral cavity and pharynx are squamous cell carcinomas of varying differentiation. Adenoid cystic and mucoepidermoid cancers arise from salivary glands. A range of histologically different cancers, such as papillary, follicular giant cell, Hürthle cell carcinomas, and lymphomas, arise in the thyroid gland.
2. Metastases. Most primary cancers of the head and neck spread by invasion of adjacent tissues and metastases to regional lymph nodes. Metastases to distant sites are infrequent. The most common location for distant metastases from head and neck cancers is the lungs.
C. Diagnosis
1. Common symptoms and signs
a. Painless mass
b. Local ulceration with or without pain
c. Referred pain to teeth or ear
d. Dysphagia, mechanical or painful
e. Alteration of speech, such as difficulty pronouncing words (tongue) or change in character (larynx, nasopharynx)
f. Persistent hoarseness (larynx)
g. Airway distress
h. Unilateral tonsillar enlargement in an adult
i. Persistent unilateral “sinusitis”
j. Persistent unilateral nosebleed or obstruction
k. Unilateral hearing loss often with serous otitis
l. Cranial nerve palsies
2. Biopsy and imaging. Primary cancers of the head and neck must be documented by biopsy. In some circumstances, epidermoid carcinoma is identified in a cervical lymph node and no obvious primary tumor can be found on physical or imaging examinations. This is described as cancers of unknown primary (CUP) or metastasis of unknown origin (MUO). “Blind” biopsies of Waldeyer ring are appropriate. Magnetic resonance imaging (MRI), computed tomography (CT), and PET/CT (positron emission tomography/CT) are used in establishing the local or regional extent of the tumor. Chest x-rays remain part of the evaluation, although intrathoracic metastases are infrequent.
3. Endoscopy. Visualization of the oral cavity, nasal cavity, nasopharynx, oropharynx, hypopharynx, larynx, cervical esophagus, and proximal trachea is essential in establishing the presence and extent of tumor. These examinations have been facilitated by development of flexible, small caliber, bright-light endoscopes. Biopsies should be done at the time of endoscopy. It is useful for all oncologists likely to be involved in the management of a specific patient to be present at the endoscopy.
4. Evaluation of masses in the neck. A new, firm, usually nontender mass or masses, in the neck, either unilateral or bilateral, especially in adults should be considered metastatic (or primary in the thyroid) cancer until proved otherwise. Before direct biopsy, search for a primary cancer is important. This search may include “blind” biopsies of Waldeyer ring and can include MRI, CT, and PET/CT examinations. Initial direct biopsy of a suspicious, enlarged cervical lymph can be done by fine-needle aspiration. Open biopsy should only be done as a last resort.
D. Staging. Staging can be based on clinical information or information found at surgery. Clinical staging is important because many patients are treated by radiation therapy (RT). Clinical staging is based on physical examination. All primary cancers must be documented histologically. If the biopsy was perfomed at another site, it is best if the pathology can be reviewed and documented at the treating institution.
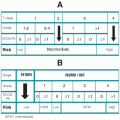
The TNM system proposed by the American Joint Committee on Cancer (AJCC) is the most frequently used system in the United States (
Table 7.1). T reflects primary tumor size and extension; N is based on the size, number, and location of cervical lymph node metastases; and M represents more distant metastases. The T staging classification varies slightly with the specific anatomic site and will be discussed with each cancer. The N and M classifications and stage groupings are the same for all head and neck cancers except nasopharyngeal carcinoma.
Stage IV is divided into three groups representing locally advanced but still resectable disease (IVA), unresectable locally advanced disease (IVB), and distant metastases (IVC).
E. Prognostic factors. The most important prognostic factors for patients with primary cancers of the head and neck are primary tumor site, size and extent, and regional or distant metastases. Histologic differentiation of epidermoid carcinomas is less important. A major risk factor is a previous head and neck
cancer. Continued cigarette smoking and consumption of alcoholic beverages expose the mucosa to known carcinogens.
F. Prevention. The main preventatives for cancer of the head and neck are abstinence from the use of alcoholic beverages and tobacco. Also avoidance or elimination of chronic irritants, such as an irregular sharp tooth or ill-fitting denture, is desirable. Isoretinoin (13-cis-retinoic acid) can reverse severe leukoplakia and possibly reduce the development of squamous cell carcinomas in the oral cavity, but it has no influence on the recurrence of previously treated cancers. New saliva-based tests are being developed to screen those at high risk for developing head and neck cancer.
G. Management principles of head and neck cancers. Before commitment to a therapy program for a specific patient, there should be input from all members of the multidisciplinary oncology group who will be involved. Often patients with head and neck cancers present before a Tumor Board where they can benefit from the combined expertise of the multidisciplinary group. Included are surgeons, radiation oncologists, medical oncologists, dentists, nurses, psychologists, social workers, and rehabilitation personnel. Proper management includes frequent, periodic examinations after treatment. Persistent or “recurrent” cancers usually can be recognized within 2 years of the completion of treatment.
1. Surgery has long been a mainstay of the treatment of patients with cancers of the head and neck. Treatment of the primary tumor requires complete removal of the tumor and its local and regional extensions. Sometimes anatomic barriers, such as the base of the skull, make such complete removal unlikely. In such situations, adjuvant RT, chemotherapy, or both may facilitate or even negate the need for radical surgery. Recent advances have promoted adequate resection of some tumors involving the base of the skull.
a. Preservation of functions, such as swallowing, voice or vision, and cosmesis, must be considered in any management plan. Tumor extension into bone, such as the mandible or maxilla, usually requires resection. Often free flap reconstruction can minimize the long-term morbidity.
b. Metastases to cervical lymph nodes, particularly from the oral cavity, paranasal sinuses, hypopharynx, and thyroid, are best treated surgically, although postoperative irradiation frequently is indicated. Removal of cervical nodes containing metastatic cancer can be accomplished by en bloc resection (radical neck dissection) or a limited procedure, such as suprahyoid dissection or modified radical neck dissection.
2. RT can control many cancers of the head and neck, usually with better consequent function and cosmesis than following radical resection. No anatomic barriers to RT exist, although specific tissue tolerance limitations do. Basic radiobiologic principles must be observed in devising specific treatment approach (see
Chapter 3).
a. Primary treatment. RT is used as the initial and possibly only therapy. This is done mainly to either preserve organs and functions or substitute for surgery for unresectable tumors.
b. Adjuvant treatment. RT is planned for use before or after surgery. The irradiated volume can be the preoperative or the postoperative tissue volume at risk, or it can be separate from the operative site, such as the treatment of cervical nodes after surgical removal of the primary tumor.
c. Volume treated. RT should include all known tumor-bearing anatomic sites plus any sites of suspected tumor spread, such as the neck in a patient with aggressive oral tongue or pharyngeal cancers.
d. RT doses. Included are incremental doses (usually in daily fractions) and total doses. Both relate to the probabilities of tumor control and treatment-related sequelae. In general, daily doses should be 180 to 200 cGy/fraction. For epidermoid carcinomas of the head and neck without surgery, the total doses are usually 6,500 to 7,500 cGy. When used as a postoperative adjuvant, the total doses can be lower (5,500 to 6,000 cGy), and when used preoperatively even lower doses (4,500 to 5,000 cGy) are appropriate.
e. Altered fractionation schemes. Special fractionation regimens have been created to exploit certain radiobiologic advantages for the treatment of head and neck cancers. (See
Chapter 3, Section III.E.) They have been tested in numerous international phase-III multicenter randomized trials, and the results have in general been favorable as compared with conventional fractionation practice, especially for locoregionally advanced disease.
f. Hyperfractionation delivers more fractions with smaller dose per fraction to a higher total dose than conventional fractionation, over the same length of overall treatment time. It aims to enhance tumor cell killing while maintaining the same level of late normal tissue damage.
g. Accelerated fractionation aims to overcome the therapy-induced accelerated repopulation of cancer cells, and delivers a conventional amount of total dose while shortening the overall treatment time with more intensely fractionated patterns.
h. Combined chemoradiotherapy. In recent years, cytotoxic chemotherapy as well as biologic response modifiers have been shown to augment the therapeutic effect of RT. Most randomized trials have shown the benefit of concurrent chemo-RT (CCRT; see Section I.J.3.), while induction or neoadjuvant chemotherapy before definitive RT continues to be tested.
i. Precision-oriented RT. Recent advances in computer technology have enabled the development of ultraprecision treatment techniques, such as
stereotactic irradiation and
intensity modulated RT (IMRT). Particle therapy with protons and heavy ions are also available in a few centers worldwide (see
Chapter 3, Sections IV.C., D.).
H. Management of the primary cancer
1. Most T1 and T2 primary cancers can be controlled equally well by surgery or RT. Therefore, the choice of treatment may be influenced by tumor site, accessibility, histologic grade, the patient’s health status, vocation, or preference. Organ or functional preservation may be provided by RT for cancers of the oral and pharyngeal tongue, floor of the mouth, larynx, orbit, or tonsil. Surgery is preferable when tumor involves bone.
2. Most T3 and T4 primary cancers often require combinations of surgery and RT. If resection is not possible, high-dose RT may still be effective and adjuvant chemotherapy may be useful. Although preoperative irradiation may reduce the tumor size and theoretically facilitate the surgery, postoperative irradiation is nearly always preferable because the extent of tumor can be better determined and tissue healing is less impaired. The total radiation doses after complete resection of the primary and regional tumors may be reduced to 5,500 to 6,000 cGy. Indications for postoperative RT include
a. Close or inadequate resection margins
b. Poorly differentiated cancers
c. Involvement of lymphatics, including cervical nodes
d. Perineural invasion
3. When cancer reappears clinically at the initial site following a complete response to the primary treatment, this is considered local recurrence of cancer. If a tumor arises at a different site, especially if the histology is different, it is considered a new cancer. The retreatment of cancers can be difficult, with reduced effectiveness and increased morbidity, although surgery may “rescue” failures of RT, and irradiation may control surgical failures.
a. Recurrence of a tumor usually indicates a biologically aggressive cancer and the prognosis is worse than before the initial treatment.
b. If the local failure is at the margin of the treatment site, it may be a direct result of “geographical miss,” and additional focal salvage treatment may still provide effective cure.
I. Treatment of metastases to cervical lymph nodes. The head and neck encompasses perhaps some of the most anatomically complicated regions of the body. Knowledge of the lymphatic system is essential in order to understand the pattern of spread of cancer in the neck. Most squamous cell carcinomas of the head and neck are at least potentially curable. Primary cancer arising in most sites in the head and neck ultimately metastasizes regionally to the cervical lymph nodes. As the status of these lymph nodes is the most significant independent prognostic factor in head and neck cancer, appropriate management of the cervical lymph nodes is essential for control of disease. Neck dissection is the standard surgical treatment for resecting cancer in the regional lymph nodes of the neck. The purpose of a neck dissection is to remove those lymph nodes involved by or at risk for involvement by metastatic cancer.
1. Types of neck dissection (ND). The Committee for Head and Neck Surgery and Oncology of the American Academy of Otolaryngology-Head and Neck Surgery reported classification of neck dissection terminology into four categories:
a. Classic radical ND removes en bloc all tissues from the clavicle to the mandible and from the anterior margin of the trapezius muscle to the midline strap muscles between the superficial layer of the deep cervical fascia (platysma) and the deep layer of the deep cervical fascia. Included are the sternocleidomastoid muscle, internal jugular vein, and accessory (11th) cranial nerve.
b. Modified radical ND usually spares the accessory (11th) nerve, the sternocleidomastoid muscle, or both. This operation is usually used when the neck is “clinically negative,” but at high risk for metastases or when the metastases to cervical nodes are minimal and when RT is to be used. A variant is the supraomohyoid dissection that removes nodes only from the upper neck.
c. Selective ND includes the possibility of dissection of supraomohyoid, posterolateral, lateral and anterior compartments, each representing a specific procedure that preserves one or more lymph node groups routinely removed in radical neck dissection.
d. Extended ND involves the removal of additional lymph node groups or nonlymphatic structures relative to the radical procedure.
2. The risk of clinically undetected metastases varies with primary tumor site and size and the histology. For example, approximately 40% of patients with squamous cell carcinomas of the oral tongue will eventually develop cervical adenopathy. This risk is higher, and often bilateral, for patients with carcinomas of the pharyngeal tongue. In contrast, cervical metastases do not develop in patients with cancers limited to the true vocal cords because there are no lymphatics.
3. Selection of treatment. When metastases to cervical lymph nodes are present at the time of diagnosis, treatment of the neck is usually dictated by the treatment modality selected for the primary tumor. For squamous cell carcinomas, primary in the oral cavity and paranasal sinuses, surgery may be preferable. When the cancers arise in the nasopharynx, RT is the choice because these tumors are radioresponsive; they often are bilateral and may not be resectable because of anatomic barriers. Other pharyngeal and laryngeal primary tumors would require both surgery and RT, but with cervical nodal metastases primary, RT with or without chemotherapy is preferable, often followed by planned ND.
4. Sentinel lymph node biopsy (SNB). In 1993, the first report was published describing the use of SNB for staging of head and neck squamous carcinoma. Even though subsequent successful studies have been performed, SNB for mucosal cancers of the upper aerodigestive tract should be evaluated only in the context of clinical trials. The place of SNB in staging and management of head and neck mucosal squamous cell carcinoma is still an ongoing debate and remains an investigational procedure. It has not achieved the status of “standard of care” for the treatment of head and neck cancer patients. It is recommended that studies of the efficacy of SNB strive, whenever possible, to segregate results of different tumor types in different head and neck locations from one another so as to produce more focused findings for discrete types of malignancies, and not group together tumor types which may in reality exhibit different biologic behaviors.
Recently, a rapid, automated quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) assay has been developed to detect lymph node metastasis in head and neck cancer with high accuracy compared to pathologic analysis and may be more accurate than intraoperative pathology. Combined, SNB and rapid qRT-PCR could more appropriately guide surgical treatment of patients with head and neck cancer.
5. Endoscopic neck dissection. Endoscopic selective neck dissection has been recently reported in patients suffering from squamous cell carcinoma of the upper aerodigestive tract located in different sites (uvula, epiglottis, and glottis). It is thought that this method may help to reduce the degree of invasiveness frequently attributed to sentinel lymphadenectomy once it has been established for head and neck cancer. At present this procedure has not achieved widespread acceptance in clinical practice.
6. Molecular approaches for the evaluation of lymph node metastases. Different methods have been used in preoperative biopsy specimens to predict neck metastases. Examples include the fraction of cancer cells immunolabeled for PCNA or Ki67, expression of E-cadherin, Snail and expression of MMP-2, MMP-7, and MMP-9, but these reports should be confirmed in larger multicenter trials.
Microarray expression profiles of more than 100 predictor genes on head and neck primary carcinomas, on the other hand, have been capable of discriminating between N+ and N0 individuals, with N0 predictive accuracy of 90%. It is highly possible that expression profiling will improve the diagnosis of nodal status, thus reducing adverse side effects related to overtreatment. The ability to predict cervical lymph node metastases based upon gene expression patterns present in a primary tumor biopsy sample would provide tremendous advantages for the determination of optimal therapeutic strategies. However, the correlations identified with the presence of nodal metastasis are inconsistent and not yet strong enough to be useful in clinical practice.
J. Role of chemotherapy in squamous cell carcinomas of the head and neck (SCCHN). Chemotherapy does not have a role in most early stage (I and II) SCCHN. The greatest benefit derived from chemotherapy is in patients with locally advanced disease when chemotherapy is used either sequentially or concurrently with RT, with or without surgery. It has been shown in this setting to increase the possibility of larynx preservation and improve survival. Data supporting adjuvant chemotherapy is largely restricted to nasopharyngeal carcinoma. In the metastatic setting, it may be used as a palliative measure and has also been shown to improve overall survival.
1. Effective agents. Many drugs have shown activity as single agents in the metastatic setting with potentially high response rates (RR) in phase II studies. Examples include methotrexate (RR 10% to 45%), cisplatin (RR 15% to 40%), bleomycin (RR 5% to 45%), 5-fluorouracil (5-FU; RR 0% to 33%), paclitaxel (RR 30% to 40%), docetaxel (RR 30% to 40%), carboplatin (RR 10% to 30%), ifosfamide (RR 25%), cetuximab (RR 16%), and erlotinib (RR 4%). These response rates often decrease significantly in phase III studies.
2. Induction chemotherapy (before surgery or RT) has been evaluated extensively. Despite very high RR, early studies did not show survival benefit with this approach. A meta-analysis has shown, however, a small but significant survival benefit when cisplatin and 5-FU are used in combination.
Several randomized studies in the United States and Europe have also shown a benefit when a taxane (usually docetaxel) is added to cisplatin/5-FU, given either before RT or CCRT. The Dana Farber group (TAX 324) showed a significant improvement in 3-year overall survival from 48% to 62% when docetaxel was added to cisplatin/5-FU, then followed by carboplatin given concurrently with RT. What is not known is whether the addition of any induction chemotherapy will improve survival compared with the optimal CCRT and studies are ongoing to address this question.
3. Induction chemotherapy regimens for SCCHN include
a. TPF, given in 21-day cycles
Docetaxel (Taxotere), 75 mg/m2, IV on day 1
Cisplatin, 75 mg/m2, IV on day 1
5-FU, 750 mg/m2/day by continuous IV infusion over 24 hours on days 1 through 5
b. TPF, also given in 21-day cycles
Docetaxel, 75 mg/m2, IV on day 1
Cisplatin, 100 mg/m2, IV on day 1
5-FU, 1,000 mg/m2/day by continuous IV infusion over 24 hours on days 1 through 4
4. Concurrent chemoradiotherapy (CCRT) has been shown to improve larynx preservation rates in intermediate, locally advanced laryngeal cancer by the Radiation Therapy Oncology Group (RTOG 91-11). A meta-analysis of CCRT used in patients with locally advanced SCCHN has shown a statistically significant improvement in overall survival (absolute improvement 8%). Survival benefits have been seen in randomized studies using various chemotherapy regimens, such as cisplatin alone, cisplatin with 5-FU, and carboplatin with 5-FU.
A study with cetuximab, a monoclonal antibody to the epidermal growth factor receptor, in combination with RT, has shown a significant survival benefit compared with RT alone (55% vs. 45% 3-year overall survival). Although CCRT using conventional cytotoxic agents will increase mucosal toxicity compared with RT alone, this increase in toxicity was not seen with cetuximab.
Although, a small (21 patients) single institution phase II study done at Memorial Sloan-Kettering Cancer Center evaluated the combination of cisplatin, cetuximab, and RT and showed an impressive 3-year overall survival rate of 76% in a group of highly selected patients with very advanced disease, this study was confounded by five significant adverse events, including two deaths. This finding was tested in a phase III cooperative group study (RTOG 05-22) of 895 patients, which showed that the addition of cetuximab did not improve either progression-free survival or overall survival (see
Ang, 2011 in
Selected Reading). Thus, single-agent cisplatin or cetuximab remains the standard of care for combination with RT for locally advanced HNSCC.
5. CCRT regimens for SCCHN include
a. Given in 21-day cycles for three cycles with RT:
Cisplatin, 100 mg/m2, IV on day 1
b. Given in 21-day cycles for 3 cycles with RT:
Carboplatin, 70 mg/m2, IV on days 1 through 4
5-FU, 600 mg/m2/day by continuous IV infusion over 24 hours on days 1 through 4
c. Cetuximab, 400 mg/m2 IV loading dose given on the week before RT starts, then 250 mg/m2 IV weekly for 7 weeks
d. Acceptable cisplatin alternative schedules for improved tolerability include 30 to 40 mg/m2 weekly, 6 mg/m2 daily, or 20 mg/m2 daily for 5 days on first and fifth weeks of RT.
e. If induction TPF is utilized, carboplatin AUC 1.5 given weekly with RT is acceptable.
6. Adjuvant chemotherapy is not recommended as standard of care after RT, with the single exception of nasopharyngeal carcinoma. In the Intergroup Study 0099, patients with stage III/IV nasopharyngeal carcinoma were randomized to RT alone or concurrent cisplatin and RT followed by three cycles of adjuvant cisplatin and 5-FU. The 3-year overall survival was 47% and 78%, respectively (P = 0.005), establishing this regimen with CCRT followed by adjuvant chemotherapy as the standard of care.
Although no benefit was seen with the use of RT alone (e.g., Intergroup 00-34) in the postoperative setting, several studies have evaluated CCRT in “high risk” patients. The two largest phase III randomized studies that have been completed were done by the European Organization for Research of Cancer (EORTC 22931) and the RTOG (RTOG 95-01). Both studies randomized patients to either high dose cisplatin as CCRT or RT alone and showed significant improvement in disease-free survival with cisplatin added. However, only EORTC 22931 showed a significant improvement in overall survival (absolute 13% survival benefit at 5 years). Subset analyses of both studies have shown that significant improvement in survival is seen only for patients with either extracapsular lymph node extension or positive surgical margins.
a. CCRT plus adjuvant regimen for nasopharyngeal carcinoma: Cisplatin, 100 mg/m2, IV on day 1 of 21-day cycles for three cycles concurrently with RT (alternatively, carboplatin AUC 6 may be substituted for cisplatin);
Followed by three 28-day cycles (after RT is completed) of Cisplatin, 80 mg/m2 IV on day 1 (or carboplatin AUC 5 if carboplatin used for combined portion), and
5-FU, 1,000 mg/m2/day by continuous IV infusion over 24 hours on days 1 through 4
b. Postoperative CCRT regimens for all other HNSCC (nonnasopharyngeal carcinomas):
(1) Cisplatin, 100 mg/m2, (or carboplatin AUC 6) IV on day 1 of 21-day cycles for three cycles concurrently with RT
(2) Standard weekly cetuximab 400 mg/m2 loading dose followed by 250 mg/m2
(3) Weekly carboplatin AUC 1.5 to 2
7. Reirradiation. The standard of care for patients with recurrent unresectable disease in a previously irradiated field is palliative chemotherapy. Several investigators, however, have evaluated the use of reirradiation concurrently with chemotherapy with overall 2- to 5-year survival rates ranging from 15% to 25%.
RTOG 99-11 evaluated hyperfractionated RT with cisplatin (15 mg/m2) and paclitaxel (20 mg/m2), both given daily for 5 days every 14 days for four cycles; treatment resulted in a 27% 2-year overall survival. However, this study resulted in an 8% grade 5 toxicity (death). Another unusual aspect is that growth factor was utilized concurrent with radiation on the days that chemotherapy was not being given. Given these uncertainties, despite the nice survival report, this approach is not recommended for routine use.
An early phase II study (RTOG 96-10) evaluated reirradiation using hyperfractionated RT with concurrent hydroxyurea (1.5 g) and 5-FU (300 mg/m2), both given daily for 5 days every 14 days for four cycles; treatment resulted in a 16% 2-year overall survival.
A phase III study to determine if this approach is superior to standard chemotherapy had to close early because of poor accrual. At this time, this highly toxic approach should be considered investigational and should not be done outside of a clinical trial or a center with extensive experience with this treatment.
8. Metastatic SCCHN is generally treated with chemotherapy alone and is not curable. Multiple chemotherapy agents have shown activity in this setting. Although combination regimens have shown superior improvement in responses compared with single agents, no randomized study has ever demonstrated an improvement in overall survival.
a. Methotrexate as a single agent (40 to 60 mg/m2 IV weekly) should be considered the standard of care because no other regimen had been shown to be superior.
b. Cisplatin/5-FU combination has been an acceptable standard largely due to an improved response rate. However, when tested in a randomized phase 3 trial versus single-agent methotrexate, despite an improved response rate (32% vs. 10%), no statistically significant difference was seen in survival (see Forastiere AA, Metch B, et al. in Selected Reading). Most practitioners reserve this regimen for younger, more fit patients who can tolerate cisplatin.
c. The roles of platin/5-FU and cetuximab
(1) The EXTREME trial was presented in June of 2007, which compared cisplatin (100 mg/m2 on day 1) or carboplatin (AUC 5 on day 1) plus 5-FU (1,000 mg/m2/day by continuous infusion on days 1 through 4), both with and without cetuximab (400 mg/m2 loading dose followed by 250 mg/m2 weekly). This study showed a significant improvement in median survival with the addition of cetuximab from 7 to 10 months. No crossover to cetuximab was allowed in the study.
(2) A randomized phase III trial by the Eastern Cooperative Oncology Group compared cisplatin and placebo with cisplatin and cetuximab in 117 eligible patients. The median and progression-free survivals were 8 and 3 months in the control group versus 9 months (P = 0.21) and 4 months (P = 0.07) in the experimental arm. Crossover was allowed in this study.
(3) Many investigators have interpreted the results of these important studies to mean that cetuximab should be used in the treatment of metastatic SCCHN. This is not necessarily so as the first-line regimen. For example, 5FU/cisplatin may be used as front line therapy with cetuximab used for second line. It is always appropriate, when available, to treat on a clinical trial, even if this means cetuximab is held until for second- or third-line treatment since the clinical studies do not clearly establish this agent for routine front line usage.
K. Adverse effects of treatment. All treatments of cancer, even when properly administered by current standards, may have unintended adverse consequences.
1. Radical surgery adverse effects depend on the primary site and the extent of ND
a. Interference with swallowing and/or speech
b. Loss or change in quality and forcefulness of voice
c. Aspiration pneumonitis
d. Shoulder or upper limb weakness as a result of resection of the spinal accessory nerve resulting in denervation of the trapezius muscle
e. Localized cutaneous sensory change or loss; injury to the cervical plexus can result in neuropathic pain and sensory loss in the anterolateral neck extending to the shoulder
f. Hypothyroidism
g. Diplopia, visual loss
h. Cosmetic deformities
2. Radiation therapy adverse effects depend on the radiation fields, dose, dose rate, technology, and whether chemotherapy is given concomitantly.
a. Acute, self-limiting effects
(1) Erythema of skin
(2) Conjunctivitis
(3) Mucositis in oral cavity, oropharynx, hypopharynx, nasopharynx, larynx, nasal fossa
(4) Alteration of taste
(5) Xerostomia, which may be minimized by total radiation dose reduction through use of techniques such as intensity modulated radiation therapy (IMRT). Medications, such as pilocarpine (Salagen), have been tried without scientifically documented success.
(6) Epilation (dose related) involving scalp, facial hair, eyelashes, eyebrows. Returning hair may be more sparse and even of a different color and texture.
(7) Edema; laryngeal edema is the most serious
(8) Lhermitte syndrome is an infrequent problem manifested as an “electric shock-like” sensation, usually in the upper limbs precipitated by flexion of the neck. This syndrome is secondary to radiation-induced change, probably temporary demyelination. It is not a precursor of permanent myelopathy.
(9) Infection, the most frequent of which is candidiasis controllable by fluconazole.
b. Long-term or permanent
(1) Xerostomia. Recovery from acute changes may be minimal with longterm adverse consequences, including tooth decay, oral infections, and problems swallowing and associated weight loss. Xerostomia also may be associated with autoimmune disorders (Sjögren syndrome), diabetes, scleroderma, and many medications, including antidepressants, antihypertensives, and medication for allergies.
(2) Altered taste: usually for salt or sweet
(3) Cataract: develops slowly (more frequent in diabetics)
(4) Osteoradionecrosis, usually of the mandible (worse with poor oral hygiene)
(5) Cervical myelopathy appears over several months and is permanent
(6) Soft tissue change: atrophy, telangiectasia, rarely ulceration
(7) Skin cancer: described in literature but is very rare
(8) Epilation
3. Chemotherapy as an adjunct can increase acute side effects of RT. The chemotherapeutic agents used for head and neck cancers will promote hematosuppression. The unique side effects of cisplatin and carboplatin are nephrotoxicity and neurotoxicity. The 5-fluorouracil commonly causes mucositis independent of RT, diarrhea, cardiotoxicity, and palmar-plantar erythrodysesthesia (the latter with infusional regimens). Taxanes can cause acute allergic reactions that usually can be prevented by pretreating with glucocorticoids, arthralgia/myalgia, and peripheral neuropathy.