Sexual Function and Pregnancy
Eric E. Prommer
I. SEXUAL FUNCTION IN PATIENTS WITH CANCER
A. Background. Sexuality is a complex and subjective concept that changes over time as a person ages and gains experience. Sexuality as a concept can include body image (how someone sees oneself physically and perceives one’s overall health and sexuality), sexual response (interest, function, and satisfaction), sexual roles, and relationships. Sexuality is a personal expression of one’s self and one’s relationship with others.
The effects of cancer and its treatment on sexuality are not usually included in assessments and plans of care for patients, nor are they often addressed in patient education. The disease and its treatments may cause patients to doubt their humanness and their passion for living; at the same time, their body image and their ability to express themselves sexually may become altered. Consequently, closeness, sharing, and other aspects of sexual expression may be avoided or neglected at a time in life when these experiences can be most beneficial. Factors affecting sexual function in cancer patients include the following:
1. Psychological factors. In the early stages of cancer diagnosis and treatment, patients may confront feelings of depression, fear of death or of treatment consequences, apprehension of functional loss, deterioration of self-esteem, or impairment of a long-lasting emotional and sexual balance with their spouse. Both patient and spouse may experience difficulties in discussing sexual relationship issues, feeling that it is not appropriate when confronting cancer. Libido is adversely affected from the initial steps of diagnosis and treatment planning, and sexually oriented thoughts and desire, if they exist, may result in feelings of guilt and further suppression of sexuality. Patients may experience fears—often unrealistic—of potential harm to themselves or their partner during sexual activity, especially when cancer treatment is ongoing. Patients must be evaluated and treated for depression.
2. Body-image alterations. Body-image changes for men and women are related to perceived losses and influences. The term influences relates to the quality of relationships before the diagnosis of cancer and the amount of control and information the patient had at the time of diagnosis. For women, losses include missing body parts (mastectomy), loss of menses, loss of sexual sensation, and, ultimately, loss of womanhood. For men, body-image changes as a result of treatment include loss of ejaculatory function, incontinence, penile deformities, and skin changes.
3. Physical symptoms. Uncontrolled symptoms impair all aspects of sexual function, including sexual interest and sexual desire. Fatigue, gastrointestinal symptoms (nausea, diarrhea), urinary tract symptoms, sleep disorders, and pain can alter sexual function. Surgical treatment, chemotherapy, radiation therapy, combined-modality treatment, and biologic and hormonal therapies may all exacerbate physical symptoms.
4. Drug effects. Chronic opioid consumption to control pain in cancer patients has been demonstrated to induce hypogonadism in men and further exacerbate depression, fatigue, and sexual ill health. In men, hypogonadism is also due to androgen-deprivation therapies or bilateral orchiectomy. Treatment of depression and anxiety in cancer patients with psychotropic drugs may further impair sexual function by adverse effects on libido, erection ejaculation, and orgasmic function. Selective serotonin-reuptake inhibitors (SSRIs) also have been reported to decrease libido in up to 40% of patients. SSRIs and tricyclic antidepressants (TCAs) have been shown to impair orgasmic function; indeed, they are used in clinical practice to treat premature ejaculation.
5. Impaired sexual response. Even before a diagnosis of cancer, women may have problems with sexual function. More than 40% of healthy women have been reported to have one or more sexual problems, such as vaginal dryness, lack of sexual interest, dyspareunia (pain with intercourse), difficulty reaching orgasm, or lack of pleasure with sexual activity. Cancer and its treatment can compound these difficulties.
6. Sexual roles and relationships. Research from cancer survivors suggests that survivors who had a good sexual relationship before therapy continued to have a satisfying relationship after surgery for breast cancer. Understanding and support from the partner were critical for the survivor to be able to obtain and maintain healthy sexual roles and relationships. The partner’s overall sexual health and function may also influence a survivor’s sexual roles.
7. Cultural differences. Research from breast cancer patients suggests that culture may affect body image. There are no data on cultural effects when it comes to male sexuality and cancer.
B. Sexual problems specific to women
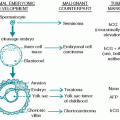
1. Germ cell depletion is discussed in Section III. Indirect indicators of menopause are amenorrhea, increased serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels, and symptoms of estrogen deficiency. Symptoms include hot flashes, loss of vaginal lubrication, atrophy of genital structures, and discomfort with intercourse.
2. Hormonal therapy for breast cancer. Tamoxifen, which is commonly used in women with breast cancer, may have a positive estrogenic effect on the vaginal mucosa or may contribute to vaginal atrophy and dyspareunia. Patients who are taking tamoxifen often experience hot flashes or vaginal discharge. Tamoxifen does appear to have a somewhat proestrogenic effect on serum lipids and bone density. Aromatase inhibitors may have a lower incidence of estrogen deficiency symptoms compared with tamoxifen, with an as yet unknown effect on general sexual function.
3. Chemotherapy may cause ovarian failure (see Section III.B). Emotional and physical changes can also adversely affect sexual function. The effect of chemotherapy on ovarian androgen output is unknown. Diminished androgen production affects libido.
4. Radiation therapy (RT). Effects of ionizing radiation on sexual function depend on age, field, and dose (see Section III.A). RT for cervical cancer leads to vaginal fibrosis, dyspareunia, and ovarian failure. Symptoms may not become apparent until 1 year after treatment.
5. Pelvic surgery
a. Cervical conization does not impair desire, arousal, or orgasm.
b. Radical hysterectomy has been shown to have no negative impact on sexual satisfaction after abdominal hysterectomy, whether subtotal or total. The only predictor of negative sexual experience of partners after hysterectomy was
negative sexual experience before hysterectomy. Women may need to experiment with different positions to experience comfortable penetration.
negative sexual experience before hysterectomy. Women may need to experiment with different positions to experience comfortable penetration.
c. Radical cystectomy can lead to decreased vaginal lubrication and dyspareunia. Newer techniques, such as quality-of-life (QOL) measurements, have shown that newer surgical procedures can maintain sexual function compared with traditional techniques. These newer approaches with cystectomy involve (1) bilateral nerve-sparing (NS) surgical technique, (2) preservation of the anterior vaginal wall (to enhance lubrication) and anterior vaginal tubularization (to preserve the depth of the vagina), and (3) avoidance of routine hysterectomy.
d. Abdominoperineal resection (APR). Sexual and bladder functions are quite often sacrificed when a conventional low anterior resection and APR with an extended lymph node dissection (LND) are performed in patients with advanced lower rectal carcinoma. These complications are due to injury of the pelvic plexus. APR commonly causes dyspareunia but orgasmic function is preserved.
The consensus is that the iatrogenic genitourinary dysfunctions are mostly caused by either a non-sphincter-sparing procedure or a non-nerve-sparing surgical approach. The practice of total mesorectal excision (TME) in rectal cancer treatment has substantially improved autonomous pelvic nerve preservation with reduction of sexual dysfunction rates.
e. Total pelvic exenteration with vaginal reconstruction results in loss of vaginal lubrication, loss of some erotic zones, dyspareunia, decreased intensity of orgasm, and the need to relearn how to achieve orgasm.
6. Mastectomy. There are consistent benefits of breast conservation or lumpectomy over mastectomy alone in preserving women’s body image and comfort with sexuality. It is clear that the type of primary surgery a woman receives for her breast cancer continues to play an important role in her body image and feelings of attractiveness, with women undergoing lumpectomy experiencing more positive outcomes than women undergoing mastectomy, with or without reconstruction. Women often feel less feminine and less physically attractive after mastectomy. About one-third experience significant anxiety or depression and are unable to enjoy or tolerate making love. A similar percentage of patients’ partners reported decreased sexual activity after mastectomy and fears of causing pain during intercourse. Men’s reactions to seeing their partner’s incision and chest wall area appear to have prognostic value: If the reaction is primarily empathic rather than negative, the prognosis for good sexual adjustment appears favorable. Women treated with lumpectomy and breast irradiation have improved self-image compared with those treated with mastectomy. Women who undergo breast reconstruction have a better body image than those who do not.
C. Sexual problems specific to men. Men treated for testicular cancer, prostate cancer, and Hodgkin lymphoma (HL) are particularly at risk for sexual dysfunction (see Section II.A). Twenty percent of surviving testicular cancer patients have reported that they have been sexually inactive; many have reported decreased sense of pleasure with orgasm, anxiety, and marital unhappiness.
1. Germ-cell depletion. Clinical indicators of germ-cell depletion include decreased testicular size, severe oligospermia or azoospermia, infertility with elevated serum LH and FSH levels, and decreased testosterone level.
2. Impotence. The reported incidence of impotence in the general population is about 10%: 8% at 50 years of age, 20% at 60 years of age, and 80% at 80 years of age. The incidence of impotence in men treated for cancer is increased, particularly for men with tumors involving the pelvis and genital
tract. Often, men emotionally relate impotence to a loss of masculinity, with attendant fear, anxiety, depression, and feelings of diminished self-worth.
tract. Often, men emotionally relate impotence to a loss of masculinity, with attendant fear, anxiety, depression, and feelings of diminished self-worth.
Temporary or permanent erectile impotence is the most common symptom of sexual dysfunction in men with cancer. Recovery of erectile function is more likely in men <60 years of age and may take months to years. Pre-existing conditions, such as diabetes, cardiovascular disease, and antihypertensive medication, exacerbate the risk for erectile dysfunction. Ejaculatory dysfunction occurs less frequently and may be due to retrograde ejaculation or dry orgasm. The presence of nocturnal tumescence is helpful in differentiating nonorganic from organic causes of impotence.
3. Systemic therapy. Fatigue, nausea, alopecia, anxiety, and other general effects of chemotherapy interact to diminish libido during treatment.
a. Chemotherapy is thought to suppress Leydig cell function, resulting in decreased serum testosterone, increased serum LH levels, and resultant loss of desire and erectile function. Chemotherapeutic agents associated with neuropathy (e.g., vinca alkaloids) can cause dry orgasm with preservation of pleasurable sensation. The effect of chemotherapy on spermatogenesis is discussed in Section II.C.
b. Hormonal therapy for prostate cancer can impair all phases of the sexual response cycle. Gonadotropin-releasing hormone (GnRH) agonists (e.g., leuprolide, goserelin) reduce serum testosterone to prepubertal levels and lead to loss of libido, difficulty with arousal, and diminished intensity of orgasm. Hot flashes may occur. In addition, flutamide and similar agents can cause gynecomastia. There is evidence that patients getting intermittent androgen deprivation have improved quality of life.
4. RT
a. Prostate cancer. RT can result in loss of erectile function in 20% to 80% of patients treated for prostate cancer. Younger men with intact sexual function before RT are most likely to regain adequate erectile function. Semen volume is also reduced with RT, leading to little or no ejaculatory fluid.
b. Testicular cancer. Patients who receive radiation to the pelvis and retroperitoneum have an increased incidence of erectile dysfunction. The effects of RT on sperm count are discussed in Section II.B. Men treated for testicular cancer had a higher risk of having low sexual desire and erectile dysfunction 3 to 5 years after completion of therapy than comparators. These sexual dysfunctions were not significantly associated with treatment intensity or hypogonadism.
c. Testicular shielding should be used if the distance between the testes and the radiation field boundary is <30 cm. Radiation dose to the testes is reduced to <10% of the total dose if this method is used.
5. Surgery. After the recovery period from pelvic surgery itself, the desire phase generally remains intact. Orgasmic function may be normal or reduced.
a. Radical prostatectomy causes impotence or impaired erection in most patients, although partial recovery of erectile function is possible. Parasympathetic stimulation causes tumescence; sympathetic stimulation causes detumescence. One or both of these autonomic bundles are at risk during radical prostatectomy.
b. Nerve-sparing techniques during radical prostatectomy allow a greater percentage of men to recover erectile function (reportedly up to 85%). Closer analysis, however, has disclosed that many men do not have erections firm enough for vaginal penetration.
c. Radical cystectomy results in erectile dysfunction and dry orgasm. With nerve-sparing procedures, up to 67% may recover erectile function.
d. APR leads to problems with erection (55%) and dry orgasm as a result of nerve damage.
e. Total pelvic exenteration results in permanent impotence and dry orgasm.
f. Retroperitoneal lymph node dissection (RPLND) leads to retrograde ejaculation. With modified RPLND in clinical stage I nonseminomatous germ-cell tumor patients, ejaculatory function can be preserved in about 90% of cases.
D. Guidelines for treatment of sexual problems