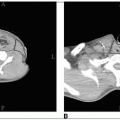
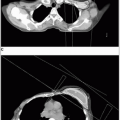
The kidneys are located in the retroperitoneal space between the eleven thrib and the transverse process of the third lumbar vertebral body; the right kidney is slightly more inferior than the left because of its relationship to the right hepatic lobe.
The kidney is enveloped by a fibrous capsule and surrounded by perinephric fat, which is surrounded by Gerota’s fascia.
The kidneys move vertically within the retroperitoneum as much as 4 cm during normal respiration (30).
The ureters course posteriorly and inferiorly, paralleling the lateral border of the psoas muscle until they curve anteriorly to join the bladder at the trigone.
The lymphatics of the kidney and renal pelvis drain along the renal vessels. The right kidney drains predominantly into the paracaval and the interaortocaval lymph nodes (LNs); the left kidney drains exclusively to the para-aortic LNs (21).
Lymphatic drainage of the ureter is segmented and diffuse and may involve any of the renal hilar, abdominal para-aortic, paracaval, common iliac, internal iliac or external iliac LNs.
Primary renal cell tumors may spread by local infiltration through the renal capsule to involve the perinephric fat and Gerota’s fascia.
The tumor may directly grow along the venous channels to the renal vein or the vena cava.
The renal vein is invaded by tumor in 21% of cases and the inferior vena cava in approximately 4% (34). The incidence of LN metastases is 9% to 27%; they most frequently involve the renal hilar, para-aortic, and paracaval LNs (10).
Approximately 45% of patients with renal cell carcinoma (RCC) have localized disease; 25% have regional disease, and approximately 30% have evidence of distant metastases at the time of diagnosis (10).
Approximately one half of the patients with RCC will eventually develop metastatic disease. Metastatic sites include lung (75%), soft tissue (36%), bone (20%), liver (18%), skin (8%), and central nervous system (8%) (22).
Upper urinary tract carcinoma is frequently multifocal; patients have a significant risk of developing tumors at several sites along the urothelium, particularly in those with large tumors or carcinoma in situ.
Ureteral tumors tend to occur in the distal third of the ureter (22).
Transitional cell carcinoma of the upper urothelial tract may spread by both direct extension and blood-borne and lymphatic metastases.
Implantation of tumor cells in the bladder may occur.
In 94 patients, none of 43 with low-grade tumors had LN metastasis, compared with three of 22 patients with grade 3 or 4 tumors (8). In a retrospective review of 1,363 patients, LN positivity increased incrementally with advancing pathological stage—less than 1% for T0/Ta/Tis, 2% for T1, 8% for T2, 17% for T3, and 46% for T4 (p< 0.001). High-grade tumors were more likely to have positive LN (15% high grade versus 2% low grade, p < 0.001) (20).
Gross or microscopic hematuria is the most frequent symptom associated with RCC.
Patients with RCC may be asymptomatic (with tumor being an incidental finding), or there may be signs and symptoms related to a local mass or systemic paraneoplastic syndromes.
Paraneoplastic syndromes associated with RCC involve parathyroid-like hormones, erythropoietin, renin, gonadotropins, placental lactogen, prolactin, enteroglucagon, insulin-like hormones, adrenocorticotropic hormone, and prostaglandins (22).
Gross hematuria, palpable flank mass, and pain describe a classic triad that occurs in only 5% to 10% of patients; it suggests advanced disease.
Gross or microscopic hematuria occurs in 70% to 95% of patients with renal pelvic or ureteral tumors (27).
Other less common symptoms include pain (8% to 40%), bladder irritation (5% to 10%), or other constitutional symptoms (5%).
Approximately 10% to 20% of patients present with a flank mass secondary to tumor or hydronephrosis (22).
Diagnostic and staging workup for RCC is given in Table 30-1.
After radiographic evaluation, in most cases, pathologic confirmation is often made at the time of nephrectomy.
Staging evaluation should include a complete history and physical examination, complete blood cell count, and liver and kidney function tests. A metastatic workup includes a chest x-ray and computed tomography (CT) or magnetic resonance imaging (MRI) scan of the abdomen and pelvis.
A bone scan should be obtained in patients with symptoms suggestive of bony metastases or an elevated alkaline phosphatase level.
PET scanning may be useful in the detection of LN or distant metastasis.
TABLE 30-1 Diagnostic Workup for Renal Cell, Renal Pelvis, or Ureter Carcinoma
General
History
Physical examination
Radiographic Studies
Standard
Chest radiograph
Intravenous pyelogram
Retrograde pyelogram (renal pelvis or ureter)
CT or MRI scan of abdomen and pelvis
Bone scan
Complementary
Renal ultrasound with color-flow Doppler
Renal arteriogram with or without epinephrine
Inferior venacavogram
CT and digital subtraction angiogram
CT of chest, brain, or other suspected organs
Laboratory Studies
Complete blood cell count
Blood chemistry profile
Urinalysis
Special Tests
Renal cyst puncture with fluid cytology (if no echinococcosis is suspected)
Endoscopic ureteroscopy
Percutaneous nephroscopy
CT of chest, brain, or other suspected organs
Urine cytology (endoscopically obtained)
Retrograde brush cytology or biopsy
If metastatic lesions are detected, histologic confirmation should be made by biopsy of either the metastatic focus or the primary tumor.
If renal vein or inferior vena cava invasion is suspected, ultrasound with color-flow Doppler may help define the extent of the tumor thrombus.
Renal arteriography is sometimes helpful in planning surgery.
The diagnostic workup for renal pelvis and ureter carcinoma is listed in Table 30-1.
Intravenous urography is frequently used to evaluate patients with renal pelvis carcinoma; a filling defect in the renal pelvis or collecting system is common.
Retrograde pyelography can be used to define the lower margin of a ureteral lesion, especially if there is significant proximal obstruction to flow of contrast from the renal pelvis.
CT or MRI of the abdomen and pelvis before and after contrast administration gives useful information about possible extension of tumor outside the collecting system.
An accurate cytologic diagnosis can be made in more than 80% of cases.
In the United States, the staging system used most commonly by clinicians is the Robson modification (29) of the Flocks and Kadesky system. The American Joint Committee staging classifications (1) for renal cell and renal pelvis and ureter carcinoma are shown in Tables 30-2 and 30-3.
TABLE 30-2 American Joint Committee on Cancer Staging Classification for Kidney Tumors
Primary Tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
T1
Tumor 7 cm or less in greatest dimension, limited to the kidney
T1a
Tumor 4 cm or less in greatest dimension, limited to the kidney
T1b
Tumor more than 4 cm but not more than 7 cm in greatest dimension limited to the kidney
T2
Tumor more than 7 cm in greatest dimension, limited to the kidney
T2a
Tumor more than 7 cm but <10 cm in greatest dimension, limited to the kidney
T2b
Tumor more than 10 cm, limited to the kidney
T3
Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond Gerota’s fascia
T3a
Tumor grossly extends into the renal vein or its segmental (muscle containing) branches, or tumor invades perirenal and/or renal sinus fat but not beyond Gerota’s fascia
T3b
Tumor grossly extends into the vena cava below the diaphragm
T3c
Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava
T4
Tumor invades beyond Gerota’s fascia (including contiguous extension into the ipsilateral adrenal gland)
Regional Lymph Nodes (N)
NX
Regional LNs cannot be assessed
N0
No regional LN metastasis
N1
Regional LN metastasis
Distant Metastasis (M)
M0
No distant metastasis (no pathologic M0; use clinical M to complete stage group)
M1
Distant metastasis
Stage Grouping
Stage I
T1
N0
M0
Stage II
T2
N0
M0
Stage III
T1 or T2
N1
M0
T3
N0 or N1
M0
Stage IV
T4
Any N
M0
Any T
Any N
M1
Source: Staging from Edge SB, Byrd DR, Compton CC, et al., eds. AJCC cancer staging manual, 7th ed. New York, NY: Springer Verlag, 2009, with permission
TABLE 30-3 American Joint Committee on Cancer Staging Classification for Renal Pelvis and Ureter Tumors
Primary Tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Ta
Papillary noninvasive carcinoma
Tis
Carcinoma in situ
T1
Tumor invades subepithelial connective tissue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access