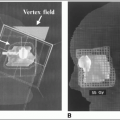
Unsealed Radionuclides: Physics and Clinical Applications
Guidelines for therapeutic use of unsealed radionuclide sources have been published by the American College of Radiology (1). Clinically used radionuclides are listed in Table 7-1.
Primary uses of nonsealed radionuclide therapy include treatment of the following: benign or malignant thyroid disease, hematologic disease, malignant bone disease, and benign or malignant disease within a body cavity (5, 8, 13).
It is mandatory to verify that female patients are not pregnant or breast-feeding at the time of oral or intravenous radionuclide therapy.
Pregnancy may be ruled out by a negative beta human chorionic gonadotropin test obtained within 48 hours before administration of the radiopharmaceutical, documented hysterectomy or tubal ligation, a postmenopausal state with absence of menstrual bleeding for 2 years, or premenarche.
Breast-feeding must be discontinued for 1 to 2 weeks before administration of a radiopharmaceutical (15).
IODINE-131 (131I)
The biologic half-life (Tbio) of iodine in normal adults is 20 to 200 days. With a physical halflife (Tphy) of 8.06 days, the effective half-life (Teff) may range from 5.74 to 7.74 days, according to the following formula:
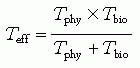
The Teff of 131I in postoperative patients with thyroid carcinoma, although not extensively investigated, is estimated to be approximately 17 hours (11).
Three strategies are used to determine the administered activity for patients with hyperthyroidism:
Empiric strategy: Most patients receive 3 to 5 mCi (110 to 185 MBq); those who are not euthyroid after 6 months receive a second administration.
Fixed-administered activity strategy: Calculated by determining a fixed activity per gram of tissue:
TABLE 7-1 Clinically Used Nonsealed Radionuclides
Radionuclide
Emitted Particles E max
Physical Half-Life (days)
Decays to
Beta (β–)
Gamma (γ)
131I
606 keV (10%)
364 keV (81%)
337 keV (7.3%)
284 keV (6%)
8.06
131Xe
32P
1.70 MeV
–
14.3
32S
153Sm
0.81 MeV
0.29 MeV
1.9
153Eu
89Sr
1.46 MeV
–
50.6
89Y
186Re
1.07 MeV (19%)
137 keV (9%)
3.8
186Os
90Y
934 keV
–
2.7
90Zr
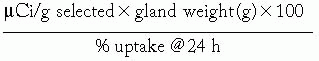
The µCi per g selected, ranging from 55 to 110 µCi per g (1.5 to 3.0 MBq per g), is based on clinical experience.
Delivered dose method: Irradiation dose of 50 to 100 Gy is selected as a target dose for the gland:

where 90 is a constant based on tissue absorbed fraction of the dose and Tbio of 24 days.
Treatment strategies for postoperative patients with thyroid carcinoma are either empiric, with administered activities of 30 to 250 mCi, or are based on the delivered dose method.
Benign Thyroid Conditions
131I for hyperthyroidism is used to treat diffuse toxic goiter (Graves’ disease), toxic nodular goiter, and solitary toxic nodule.
A recent radioiodine thyroid uptake is necessary; the size of the thyroid gland should be estimated by palpation or some other means.
The patient’s system should be free of iodide-containing medications, iodine contrast agents, exogenous thyroid hormone, and antithyroid medications.
Usual initial absorbed doses are 50 to 200 µCi (1.85 to 7.40 MBq) per g of thyroid (after adjusting for current 24-hour radioiodine uptake).
For hyperthyroidism, the usual dose is 3 to 5 mCi (110 to 185 MBq).
For toxic nodular goiter, doses of up to 30 mCi (1,110 MBq) typically are used; however, higher doses may be necessary for large multinodular glands (1).
Current Nuclear Regulatory Commission (16) regulations require hospital confinement if the patient’s body contains 30 or more mCi (1,110 MBq).
Thionamides (propylthiouracil, methimazole) inhibit organification of iodide; if 131I therapy is administered during the first 2 weeks after discontinuing thionamide, the dose may need to be increased.
If the patient does not adequately respond to a dose of 131I, subsequent treatments may be given, at least 2 months after, to allow for the full effect of the initial treatment to occur.
Malignant Thyroid Conditions
Ablation of Thyroid Remnant
Thyroid hormones should be depleted so that level of serum thyroid-stimulating hormone is elevated; this is done by withholding thyroid hormone replacement (4 to 6 weeks for thyroxin, 2 weeks for triiodothyronine) after surgery.
All routine blood work should be performed, and laboratory specimens obtained, before treatment.
If the remnant is large, thyroid scintigraphy with technetium-99m (99mTc) (pertechnetate) or iodine-123 (123I) may be used to determine the thyroid remnant uptake of radioiodide. For a very small remnant, a whole-body survey with 131I may be useful.
Usual oral doses of 131I (sodium iodine) are 100 to 150 mCi (3,700 to 5,500 MBq).
Side effects include radiation gastritis, radiation sialitis, and diarrhea. With larger or multiple doses, xerostomia may rarely occur.
The patient must be isolated from others and placed on radiation precautions until radioactivity is equivalent to 29.9 mCi (1,100 MBq) or less and the exposure rate at 1 m is lower than 5 mR per hour.
On May 29, 1997, the NRC promulgated new rules that allow administration of 131I for thyroid diseases on an outpatient basis. The new regulation is contained in Section 10, Code of Federal Regulations, Part 35 (10 CFR 35), and the specific changes are noted in Section 35.75 (15).
Activity below which the patient can be discharged, depending on fractional thyroid uptake, is shown in Table 7-2.
TABLE 7-2 Postthyroidectomy for Thyroid Cancer
Fractional Thyroid Uptake of 131I
Administered Activity Below Which Patient Can be Released (mCi)a
0.025
257
0.035
241
0.05
220
0.075
193
0.10
171
0.125
154
0.15
140
0.175
128
0.20
119
0.225
110

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access