26 Understanding the strengths and weaknesses of clinical research in cancer
What are the important elements when assessing reports of trials?
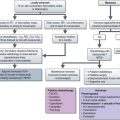
By far the most important elements are the design and conduct of the trial. The methods of analysis, while still important, are generally less likely to lead to inappropriate conclusions than inappropriate design and management of a trial. This chapter is based on the methods recommended by various EBM groups (Box 26.1).
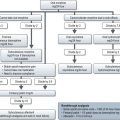
Because there is such a huge volume of medical literature published each year, it is important to take a systematic approach to what you appraise thoroughly (Box 26.2). The first step in appraisal is to screen the paper to see if it is worthy of careful reading. It may be possible to answer these screening questions on reading the title and abstract.
Box 26.2
Systematic method of research appraisal
Step 1 – Screening questions
Step 2 – Appraising a paper reporting a trial
There are a number of crucial questions that need to be answered (Box 26.2):
Concurrent or historical controls?
Did the authors use randomization?
The purpose of randomization is to ensure, as far as possible, that all factors (known and unknown) that may influence the treatment outcome are balanced between the treatment groups. This is done to reduce the risk of a chance bias. Randomization requires the use of a random device; this is normally a table of random numbers. Systematic allocation; i.e., alternating between treatments, odd or even birth date or hospital number is not an acceptable method, though it is sometimes referred to as pseudo-randomization. Since the researchers know which treatment each person is allocated to before they consent, selective allocation can occur, which will skew the results. Hence the process used for randomization must ensure that neither the trial subjects nor the investigator can influence the treatment arm each person ends up in (‘allocation concealment’).
2 Was the study based on a pre-specified protocol?
When reading a paper bear in mind the following elements of trial design:
Subgroup analysis
Where a subgroup is found to behave differently, you should consider if there is a plausible biological mechanism for this and whether other trials have found a similar finding. Where there is a statistical analysis, this should be done as a formal test of interaction. Strong empirical evidence suggests that post hoc subgroup analyses often lead to false positive results (Example Box 26.2).