Fig. 1.1
Schematic diagram illustrating molecular pathways implicated in HPV- and non-HPV-associated penile cancer. Reprinted with permission from [57]
In these situations, the E7 protein instead complexes with pRb which leads to unregulated dissociation of E2F and protein expression. Normal regulatory mechanisms in place involve an increase in p53 expression as a response to increased proliferation, which ultimately increases cell degradation. However, in the HPV proliferation cycle, particularly high risk HPV types such as HPV 16, E6 instead forms an ubiquitin ligase which leads to p53 degradation and prevention of subsequent cell degradation [31]. An additional consideration between low risk and high risk HPV types is the expression of p21 and p27 kinase inhibitors. If present in sufficient quantity, they will bind with E7 and other cyclin proteins, rendering them inactive [34]. In high risk forms, this is believed to be overcome by the high levels of E7 protein present in the viral genome [31]. An additional mechanism of cell proliferation in high-risk HPV types involves E6 independent mediated proliferation via its terminal PDZ binding domain. E6 is believed to mediate cell proliferation and may be important in the metastatic potential of some HPV-related neoplasms [35].
The progression to malignancy requires a complex interplay between continued viral genome expression, packaging, and release to promote infection. Some theories suggest that the progression to malignancy occurs after uncontrolled cell proliferation, which ultimately leads to continued point mutations and ultimately carcinogenesis. However, this remains an area of debate and continued research.
HPV-Independent Pathway
While non-HPV-related penile cancers may be managed in similar manners as their HPV counterparts, they indeed represent a separate entity in regard to pathophysiologic mechanisms. While the understanding of HPV-mediated penile cancers is more robust, HPV-negative carcinogenesis is less understood.
Lichen sclerosus and leukoplakia are two commonly noted HPV-negative precursor lesions associated with SCC development. Lichen sclerosus is typically associated with chronic infection, trauma, or inflammation and is also noted to have an association with urethral stricture disease [36]. Progression to SCC is noted to occur between 5 and 10 % of patients, and yearly surveillance is recommended [3]. Conversely, while leukoplakia is also associated with chronic inflammation, the rate of progression to malignancy is higher, affecting approximately 15–20 % of patients [2].
From a molecular standpoint, HPV-negative penile cancers are typically associated with p53 mutations, and this itself has been identified as being a negative prognostic factor [37]. Via p53-induced mechanisms, cell cycle inhibitors including cyclin p21 and D1 are normally induced to delay or terminate replication. However, mutational effects on p53 itself ultimately lead to uninhibited cell proliferation and malignant transformation. However, despite the involvement of these cyclins as well as other identified proteins including Ki67 (a nuclear matrix protein involved in the cell cycle), they have not yet proven to yield prognostic value similar to that of p53 [38].
Ongoing research continues to evaluate other possible HPV-negative etiologies on a molecular level. These include evaluation of the mTOR-mediated cell cycling pathways as well as those involving the human epidermal growth factor family. An additional area of focus is on the involvement of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) in penile carcinogenesis, related to their role in the inflammatory process. In fact, mouse studies have identified increased amounts of these molecules in tissue samples representing invasive SCC as well as lymph node metastases [39].
Penile Cancer Precursor Lesions
The following section will focus on the pathophysiology of penile cancer and its preneoplastic lesions. Several penile SCC precursor lesions, often referred to as penile intraepithelial neoplasia (PeIN), can be classified as either HPV related or non-HPV related (Fig. 1.2).
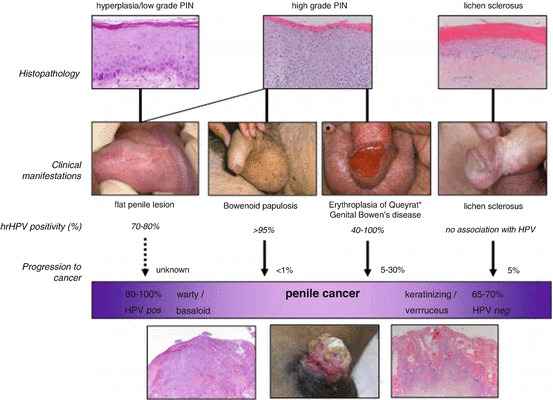

Fig. 1.2
Relationship between histology, HPV presence, clinical manifestation, and putative transformation of penile precursor lesions into penile cancer. Reprinted with permission from [58]
Condyloma Acuminatum
Condyloma acuminatum refers to nontender wart like or papillary frondular lesions that are better known as genital warts. These lesions are typically caused by HPV types 6 and 11 and are acquired by direct skin-to-skin contact. They can occur anywhere on the external genitalia but are most common on the glans and penile shaft [40]. Condyloma acuminatum has a very low risk of conversion to invasive penile carcinoma; although in a cohort of 200 cases, Cubilla et al. detected solely low-risk HPV types in 6 % of tumors [41]. Routine biopsy is not recommended for condyloma acuminatum unless lesions are atypical, pigmented, indurated, fixed, or ulcerated. Although there is a small chance of cancer progression with low-risk HPV types, the likelihood of progression is low and Condyloma acuminatum should be treated as a benign lesion.
Giant Condyloma Acuminatum (Buschke-Lowenstein Tumor)
First described in 1925 by Buschke and Löwenstein, the giant condyloma acuminatum or Buschke-Lowenstein tumor is a rare manifestation of condyloma acuminatum. It is characterized by the development and slow-progression of exophytic, cauliflower-like masses in the genital or anorectal region that infiltrate adjacent tissues [42]. These verrucous carcinomas are usually attributed to HPV types 6 and 11. Biologically, giant condyloma acuminatum is characterized by a low incidence of metastases but does have a high rate of recurrence [43]. Radical excision is recommended for complete histological examination. Other less invasive treatment options include radiotherapy, laser therapy, and topical therapy [44].
Bowenoid Papulosis
Bowenoid papulosis is characterized by red-brown pigmented popular lesions on the glans or shaft of the penis. It is typically seen in circumcised men and is diagnosed in younger patients, on average between 20 and 30 years of age. HPV 16 is most intimately associated with Bowenoid papulosis; however, multiple other HPV types have been implicated [45]. Histologically, Bowenoid papulosis is characterized by full thickness cytological atypia, which make it very difficult to distinguish from SCC in situ [25]. Bowenoid papulosis is generally considered benign; however, as it is associated with high-risk HPV types, there have been reports of it progressing to invasive cancer, especially seen in immunocompromised patients [46]. Treatment options include surveillance, topical therapy (5-florouracil), and ablation.
Lichen Sclerosus (Balanitis Xerotica Obliterans)
Lichen sclerosis, also known as Balanitis xerotica obliterans or BXO, is characterized by flat white patches on the prepuce, glans, urethral meatus, or fossa navicularis. Its mechanism of disease is unknown, but it is thought to be caused by chronic infection, trauma, or inflammation. Lichen sclerosus is often asymptomatic, however and can be associated with phimosis, meatal stenosis, and urethral stricture as the disease progresses [47]. Infrequently (4–8 %) lichen sclerosis will progress into SCC of the penis [47, 48]. Diagnosis is made by skin biopsy and should be considered in specific cases to exclude subclinical carcinoma in situ or invasive penile cancer [49]. Asymptomatic lichen sclerosus requires no treatment, while symptomatic lichen sclerosus can be treated with topical/intralesional steroids. Phimosi s can be treated with circumcision, and meatal or urethral strictures should be treated accordingly. Excision of lichen sclerosus is not recommended, as recurrence rates are typically high.
Carcinoma In Situ (Erythroplasia of Queyrat and Bowen’s Disease)
Erythroplasia of Queyrat and Bowen’s disease both refer to forms of squamous intraepithelial neoplasia with a high rate of progression to invasive SCC. Progression rates to penile cancer are cited as high as 10–33 % [3], with Erythroplasia of Queyrat tending to have a slightly higher chance of progression [50]. The main difference between the two lies in the location of the lesion; Erythroplasia of Queyrat refers to carcinoma in situ (CIS) of the glans or prepuce, while Bowen’s disease signifies CIS of the penile shaft or of the remainder external genitalia. Erythroplasia of Queyrat is typically described as well-marginated plaques with a red, velvety appearance, while Bowen’s disease is characterized by sharply defined plaques of scaly erythema. Both are generally asymptomatic. Like most SCCs of the penis, HPV types 16 and 18 play an important role in the pathophysiology of CIS of the penis. Prior to treatment, adequate biopsies must be performed to rule out invasion. When CIS is confirmed, treatment modalities include wide local excision if isolated to a discrete site, topical therapies (5-fluorouracil or 5 % imiquimod), and laser ablation (although typically less successful).
Histologic Subtypes
As previously noted, penile cancer can manifest histopathologically as several different subtypes: basaloid, warty, papillary, verrucous, sarcomatoid, adenosquamous, and mixed [8]. Verrucous subtype is a low-grade, slow-growing non-HPV-related subtype that represents 3–8 % of penile SCC [41, 51–54]. These tumors have varying gross features but often have a cobblestone or filiform morphology and histologically show variations of squamous differentiation [55]. Papillary subtype is a low-grade, non-HPV-related group encompassing verruciform histology not otherwise specified that represents 5–15 % of penile SCC [41, 51–54]. Morphologically, these tumors are large, exophytic lesions with hyperkeratosis and papillomatosis with fibrovascular cores on histology [55]. Papillary subtype is often associated with lichen sclerosus and differentiated PeIN [56]. Warty subtype represents 7–10 % of penile carcinoma and is typically associated with the HPV-related pathway [41, 51–54]. Morphologically, they produce exophytic white or gray tumors. These tumors are typically slow-growing and low grade. Histologically the tumor invades stroma in a jagged irregular fashion [55]. Basaloid subtype represents 4–10 % of penile SCC and is also related to the HPV-related pathway [41, 51–54]. It typically arises in the glans and follows an aggressive course [41]. These typically present as tan ulcerated lesions and have nests of basaloid cells histologically [55]. Sarcomatoid subtype is a less common, aggressive subtype found in 1–3 % of penile SCC with high associated mortality and recurrence [41, 51–54]. Morphologically, these tumors are bulky, polypoid masses with characteristic spindle cell histology [55].
Conclusions
SCC of the penis is a rare disease. Specific risk factors have been identified, and HPV has been proven to play a pivotal role in a significant subset of cases. Precursor lesions demonstrate variable risk to progression to invasive cancer depending upon the lesion itself, with CIS lesions carrying the highest risk of progression.
References
1.
2.
3.
4.
Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol. 2010;57:1002–12.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree