Chapter Outline
Development of Brain Structures
Genetic and Signaling Pathways Implicated in Development and in Pediatric Tumors
CONCEPTUAL ORGANIZATION OF PEDIATRIC BRAIN TUMORS
Pineocytomas and Pineoblastomas
Supratentorial or Central Nervous System Primitive Neuroectodermal Tumors
Atypical Teratoid Rhabdoid Tumors
Tumors of the central nervous system (CNS) account for approximately 25% of pediatric cancer but are now the leading cause of cancer-related mortality in children. The complexities of tumors in this site are related to the large number of different histologies within the CNS and a historic nomenclature that is confusing, even to those in this field. With the need to modify therapies to spare important neurocognitive function in the youngest patients and the presence of the blood-brain barrier (BBB), which restricts the delivery of effective therapies, improvement in outcome has lagged well behind that of many other cancers, especially childhood leukemia. The molecular revolution offers the chance to begin classifying tumors by the signals that drive their phenotype rather than by their appearance under the microscope. In this chapter we will discuss the different types of brain tumors in children, their diagnoses, and their treatments, while incorporating the expanding knowledge of tumor biology.
Although clinical studies often focus on progression-free survival (PFS) and overall survival (OS), successful therapy incorporates much more. Accepting a lower overall cure rate but preserving neurocognitive function is the norm for many types of brain tumors, especially those of infants and young children. Optimization of outcome requires expertise in multiple subspecialties that play a role in the care of these children. The skill of the neurosurgeon, the sophistication of the radiation planning, and the safe administration of chemotherapy are all important factors in improving the long-term outcome for these patients. In fact, many centers now use neurooncologists who have completed additional training in outcome optimization. When combined with a large number of subspecialty services (e.g., endocrinology, neurology, neuropsychology, social work, back to school, and physical and occupational therapy), truly optimal care is now possible for this patient population. Needs of the family continue to evolve as patients transition from diagnosis to treatment to posttherapy follow-up. A comprehensive understanding of pediatric neurooncology and the delivery of comprehensive care to these patients and their families will be the focus of this chapter.
To assist the reader, a number of important review articles summarizing different aspects of the care of children with CNS tumors are referenced.
Epidemiology
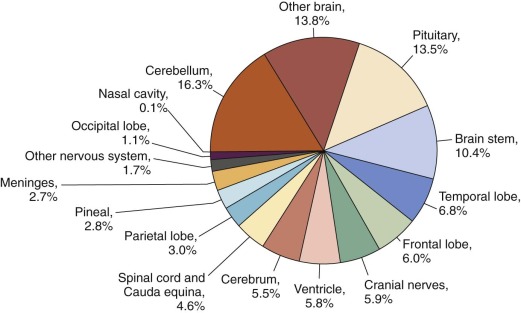
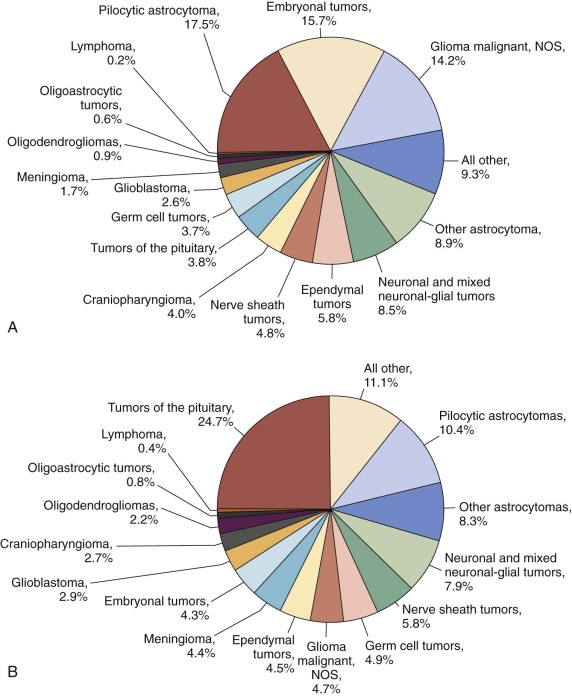
Primary CNS (PCNS) tumors rank second behind leukemia as the most common pediatric cancer diagnosed in the United States each year ( Fig. 57-1 ). Brain tumors are the most common form of solid tumors in children and are now the leading cause of death from solid tumors in children. The spectrum of adult brain tumors based on location, histology, and outcome differs significantly from that in pediatrics, suggesting that the causative events are different from those for pediatric brain tumors. No single standard classification system has been implemented for pediatric brain tumors, although development of a standardized platform for epidemiologic studies has been attempted. The 2013 Central Brain Tumor Registry of the United States Statistical Report included primary nonmalignant (1.93 per 100,000 children) and malignant (3.33 per 100,000 children) pediatric brain and CNS tumors with a total incidence of 5.26 per 100,000 children. Similarly in the 2013 National Cancer Institute Surveillance Epidemiology and End Results (SEER) Cancer Statistics Report, the annual age-adjusted incidence rate of pediatric malignant brain and other nervous system tumors was listed as 3.1 cases per 100,000 children. The rate is higher in males (3.2 per 100,000) compared with females (2.9 per 100,000). Approximately 4100 new cases of childhood PCNS tumors are diagnosed in the United States each year. Of these, an estimated 3007 will be in children younger than 15 years. The incidence for all brain tumors is highest among 0- to 4-year-olds (5.77 per 100,000) and lowest among 10- to 14-year-olds (4.78 per 100,000), results that are similar to those reported in 2005. The age-adjusted mortality rate for pediatric CNS tumors in 2010 was 0.6 per 100,000 children, resulting in an estimated 500 deaths per year in the United States for those aged 0 to 19 years. The prevalence rate for all malignant and benign pediatric CNS tumors (ages 0 to 19 years) is estimated at 9.5 per 100,000, with more than 26,000 children estimated to be living with this diagnosis in the United States in 2000. However the prevalence rate for patients with only malignant brain tumors was 7.9 per 100,000, with more than 21,000 children estimated to be living with a diagnosis of primary malignant CNS tumor in the United States in 2000. The distribution of pediatric brain tumors by site is presented in Figure 57-2 . Different brain tumor histologies have different age distributions ( Fig. 57-3 ). The most common histologies in the younger age group (ages 0 to 14 years) include pilocytic astrocytomas (PAs) and medulloblastomas, which account for 20% and 16% of cases, respectively. The broad category of glioma accounts for 56% of tumors in children younger than 15 years. The most common histologies in adolescents ages 15 to 19 years include PA and pituitary tumors, which account for 15% and 14% of cases, respectively. The broad category of glioma accounts for 45% of tumors in adolescents ages 15 to 19 years. The rates among boys are slightly higher than those in girls, and brain tumors are more common in whites (4.7 per 100,000) than in blacks (3 per 100,000).

The histologic-specific differences in brain and CNS tumor distribution by age and gender suggest that childhood tumors have different mechanisms whereby normal cells, possibly somatic CNS stem cells, are susceptible to oncogenic mutation. Although certain histologic subtypes can also differ by race, the overall concordance of tumor histologies among different ethnic groups and different locations suggest that specific local environmental factors are not the cause of most cancers in children. The incidence of common pediatric brain tumors such as medulloblastoma, malignant gliomas, and diffuse pontine glioma do not differ significantly in industrialized versus nonindustrialized countries, in vegetarian versus meat-eating societies, and in areas where smoking and drinking are permitted versus where they are not permitted. Similarly death rates for children with CNS tumors between different ethnic groups within the United States (Hispanics, Asians, blacks, and whites) do not differ significantly for most CNS tumor types.
In the mid 1990s an increase in the incidence of childhood brain cancer appeared to occur compared with that in the previous two decades. This increase is now thought to reflect the introduction and widespread use of magnetic resonance imaging (MRI) technology in the mid 1980s, resulting in improved detection and reporting of pediatric brain tumors. More precise classification of brain tumors and diagnostic capabilities, such as stereotactic biopsy, also may have contributed to the increase in incidence. The rise in incidence was followed by the establishment of a new baseline that has remained stable. Mortality rates have not mirrored the increase in incidence.
PCNS tumors develop from an accumulation of genetic changes. Such changes can result from inherited mutations or develop from exposure to chemical, physical, or biologic agents that damage deoxyribonucleic acid (DNA). Unlike in adults, for whom lifetime exposure is significant, most pediatric tumors are believed to be the result of random genetic mutations that occur during normal cellular proliferation. Today molecular biologic techniques are used to unravel the complex genetic errors that lead to the development of CNS tumors. To date most pediatric brain tumors have demonstrated a limited number of mutations, even in highly aggressive tumors, suggesting that their presence during development or within the stem cell compartment is an important aspect of tumorigenesis in pediatric patients.
The search for causative factors that place children at risk for developing CNS tumors has not yielded clear answers. Numerous epidemiologic studies have evaluated potential risk factors. Similar to most pediatric cancers, no specific risk factor explains more than a small proportion of tumors. Factors studied but not conclusively found to increase risk include exposure to tobacco and smoke, alcohol, traffic-related air pollution, electromagnetic fields, pesticides, and occupational and industrial chemicals; diet; drugs and medications; infections and viruses; epilepsy; and consumption of cured meats during pregnancy. The dramatic increase in the use of cellular telephones has generated concerns about the potential risk for the development of brain tumors. A meta-analysis of nine case-control studies concluded that cellular phone users have no overall increased risk of brain tumors. The potential risk after long-term cellular phone use awaits analysis in future studies. A seasonal variation unique to medulloblastoma incidence by month of birth may provide evidence for an environmental exposure cause, although further studies are needed. An association between atopic disease and a reduced risk of glioma has been observed in adult epidemiologic studies, with the implication that heightened immune surveillance decreases the risk of brain tumor development. Prenatal multivitamin use has been associated with a protective effect in the development of pediatric brain tumors in a large meta-analysis. Confirmation of this result in a prospective trial is needed.
The role of viruses in the pathogenesis of tumors has been documented in experimental animals, and adenovirus serotypes have been shown to induce tumors in rodents. Adenoviral sequences were evaluated in more than 500 tumors derived from 17 different pediatric cancer entities. Although most leukemias and solid tumors were negative for the presence of adenoviral sequences, tumor material from 25 of 30 glioblastomas, 22 of 30 oligodendrogliomas, and 20 of 30 ependymomas, as well as normal brain, were positive by polymerase chain reaction assay for adenoviral gene sequences. This finding raises important questions about the contribution of this infectious agent to pediatric brain tumorigenesis. In contrast, tests for polyomavirus sequences in adult and pediatric CNS tumors were rarely positive.
Ionizing radiation, immunosuppression, and certain hereditary genetic disorders are the only factors that have been proven thus far to increase a child’s risk for CNS malignancy. Ionizing radiation exposure is a well-documented cause of brain tumors. Children who undergo therapeutic irradiation to the CNS for the treatment of malignancy are at risk for development of a second tumor, specifically meningioma, high-grade glioma (HGG), or sarcoma. Since its introduction in the 1970s, computed tomography (CT) has become an essential tool in the diagnosis and monitoring of disease. The growing use of CT scans has raised concerns about potential risks. Pediatric CT scans may result in a small but not negligible increased lifetime risk for cancer mortality.
Immunocompromised children are at increased risk for PCNS lymphoma. The risk for developing CNS lymphoma is 1% to 5% higher for adults and children undergoing transplantation and for those with congenital immunodeficiencies. The risk is 2% to 6% higher for persons with acquired immunodeficiency syndrome (AIDS). This risk will probably increase with longer survival because of improved AIDS treatment.
In summary although a few environmental factors are associated with an increased risk of developing a pediatric CNS tumor, the vast majority of patients have no easily identifiable risk factors. For a small percentage, inherited genetic mutations contribute to the onset of these tumors, but for the remainder, CNS tumors are likely the result of spontaneous mutations.
Neurodevelopment
Our understanding of the steps in hematopoietic development, together with the pathways and markers that distinguish precursors along each blood cell lineage, has been instrumental in allowing better classification and subsequently better treatment of leukemias. In the same way that leukemias can be viewed as deregulated expansion of hematopoietic precursor cell pools, pediatric brain tumors may similarly be considered as the proliferation of neuropoietic precursors. Thus knowledge of the steps and intermediates in neural development may help us understand and treat pediatric brain tumors. A current schema for neuropoiesis relies heavily on models for hematopoiesis but introduces two additional aspects of developmental regulation, the first of which is the importance of regionalization. During neural development a rostral-caudal gradient delineates distinct zones for proliferation and differentiation, while the orientation of proliferating cells relative to the dorsal-ventral axis provides the basis for determining the progeny of each proliferative event.
The second aspect of neuropoiesis is the concept of mitogenic niches. Although hematopoietic stem cells can proliferate and give rise to the entire array of blood cell types when exposed to the environment of the immature or mature bone marrow, neural stem cells use a number of distinct niches, some of which are eliminated before birth, some that persist through early childhood, and some that remain extant into adult life. These niches provide important cues for proliferation and differentiation but may also provide an environment that fosters the growth of tumor cells.
Neural Tube
The nervous system develops as a specialized zone of the epithelium. In the third week after fertilization, the midline zone of the epithelium becomes specialized as the neural plate. This distinctive zone extends from the caudal to the rostral portion of the embryo. While the embryo turns, the neural plate grows and folds ( Fig. 57-4 ). Subsequent fusion of the folds creates a discrete neural tube that zips up from both the top and bottom. The two last places where the fold fuses are the hindbrain (the incipient cerebellum) and the lumbar spine. Cells at the crest of the developing neural tube are the neural crest cells, which give rise to the peripheral nervous system, including sympathetic ganglia, dorsal root ganglia, and Schwann cells, as well as to melanocytes in the developing skin. The neural crest cells are the precursors to neuroblastomas, neurofibromas, and melanomas.
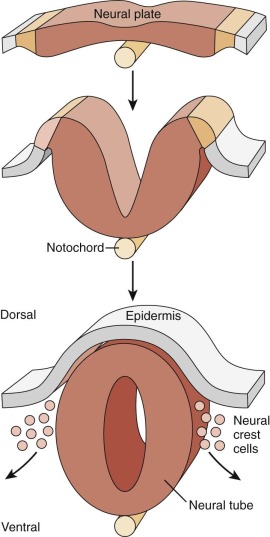
After the neural tube fuses, rapid expansion of cell number continues, but this expansion occurs very differently along the rostral-caudal axis. The dramatic expansion of cell number in the rostral neural tube provides the building blocks for the brain, whereas the more caudal regions undergo more limited growth and engender the spinal cord.
Development of Brain Structures
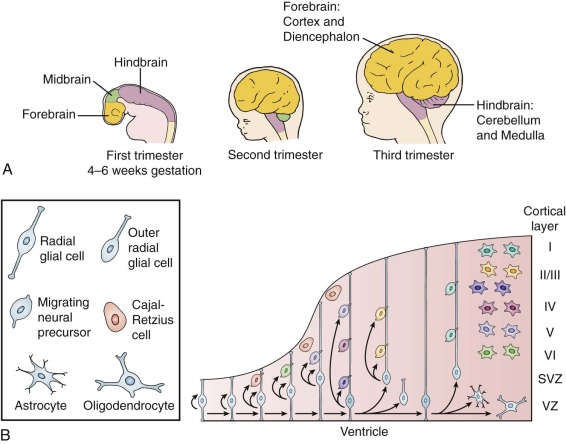
Along the rostral-caudal axis of the neural tube, three outpouchings can be seen at the end of the fourth week after fertilization—the forebrain, midbrain, and hindbrain ( Fig. 57-5 ). Subsequent branching of the forebrain forms two lateral protrusions that are destined to become the left and right cortex, and the midline portion of the forebrain gives rise to the thalamus. The midbrain does not undergo much expansion, but the hindbrain undergoes massive proliferation to give rise to the cerebellum and underlying pons, as well as the medulla. Cerebral cortical tumors, including supratentorial primitive neuroectodermal tumors (CNS PNETs) and cortical and subependymal astrocytomas, all derive from the forebrain. Posterior fossa tumors, including the distinctive pontine gliomas, medulloblastomas, and cerebellar PAs, all derive from the hindbrain. Thus the areas of the brain that undergo rapid expansion in early life engender most pediatric brain tumors. Along the dorsal-ventral plane of the neural tube, greater proliferation of neural tube precursors occurs in the dorsal part of the tube than in the ventral tube. Proliferation of more dorsal precursors gives rise to the multilayer structures of the cerebral and cerebellar cortex; these areas are also the regions that give rise to many pediatric brain tumors.
Forebrain and Cerebral Cortex
In the forebrain the open spaces within the lateral outpouchings of the neural tube become the lateral ventricles, and proliferation largely occurs adjacent to the ventricular zone (VZ) in the medial and lateral ganglionic eminences, in the subventricular zone (SVZ) or subependymal zone. Early in development, neural stem cells/precursors divide extensively to provide the cellular elements of the cerebral cortex, thalamus, and basal ganglia (see Fig. 57-5, A ).
Although the VZ in the forebrain initially contains the dividing neural stem/progenitor cells, during development the size of the VZ progressively decreases while the adjacent SVZ increases in size. The early generated cells migrate radially from the VZ and SVZ to become the neurons of the innermost cortical layer (layer VI), and later mitoses give rise to neurons that also migrate radially and occupy increasingly superficial layers. Inhibitory neurons are derived from precursors in the medial ganglionic eminence. They migrate tangentially throughout the cortex. Thus the cortex develops in an inside-out pattern. Toward the end of the prenatal neurogenic phase, the precursors of the VZ and SVZ generate glial cells, including astrocytes, oligodendrocytes, and ependymal cells.
A small population of precursors remains in the SVZ, just above the ependymal cells, throughout life. These neural stem cells continue to generate glial cells and oligodendrocytes and also continue to give rise to a limited number of neuronal cells through adult neurogenesis. An additional zone of adult neurogenesis is located in the hippocampus, adjacent to the granular zone. Thus the neural stem cell represents a common precursor for glial and neuronal cells. Accumulating data suggest that many, if not most, brain tumors arise from such stem cells or their early derivatives.
Cerebellar Cortex
The cerebellar cortex develops in a way that has some similarities to, but some differences from, the pattern in the cerebral cortex. The hindbrain is the site where the neural tube closes last. While closure occurs, the neural folds pucker to form the rhombic lips. These two protrusions will give rise to many cell types of the cerebellum and pons. Cells in the VZ of the upper rhombic lip proliferate and then begin an unusual migration pattern. The precursor cells of the rhombic lip migrate over the top of the rhombic lip and disperse by moving from the caudal aspect of the pucker to cover the rhombic lip or incipient cerebellum. The rhombic lip–derived precursors settle in a zone that covers the developing cerebellum, which is called the external granule cell (external germinal cell [EGL]) layer. This layer constitutes a site of extensive postnatal proliferation and is a specialized mitogenic niche where precursors divide and give rise almost exclusively to cerebellar granule cells, the most numerous neuronal cells in the brain. This secondary proliferative zone may be necessary to generate this vast number of granule cells. To generate the 60 to 80 billion granule cell neurons, the granule cell precursors in the EGL undergo many rounds of cell divisions, beginning in the ninth week after fertilization and continuing through the first 18 months of life in humans. Other stem/precursors in the VZ follow distinct differentiation paths. Radial glia adjacent to the cerebellum provide one source of these multipotential stem/precursors. Some VZ stem/precursors migrate toward the cerebellar white matter, where they can give rise to cerebellar interneurons and glia. Others migrate past the cerebellar white matter and form the Bergmann glia of the cerebellar cortex.
It has been suggested that four distinct subtypes of cerebellar stem/precursors each give rise to a distinct subtype of medulloblastoma. A great deal of evidence has indicated that the granule cell precursors of the EGL are a cell of origin for the sonic hedgehog (SHH) subtype of medulloblastoma. First, in very young children, medulloblastomas are often continuous with the EGL, and an intermediate zone of dysplastic cells can sometimes be seen to join the EGL and tumor tissue. Second, the appearance and pattern of gene expression of the granule cell precursors resemble those of SHH-type medulloblastoma. Finally, SHH signaling pathways that normally regulate proliferation of granule cell precursors are constitutively active in one type of medulloblastoma (discussed later).
The precursor cells adjacent to the ventricle that resemble radial glia have been suggested to be the cells of origin of Wingless (Wnt)-subtype medulloblastomas. In mouse models, activation of the Wnt pathway and/or activation of phosphoinositide 3′ kinase (PI3K) in these radial glial precursors can mimic this group of medulloblastomas.
The cellular origin and the oncogenic mutations responsible for initiating other medulloblastoma subtypes have also been identified. For example, it has been suggested that the stem cells of the white matter and earlier stem cells may provide the cellular origin for group III and IV medulloblastomas, respectively. It has been suggested that less specialized stem cells can give rise to medulloblastomas. Indeed molecular characterization of medulloblastomas suggests that genetically distinct tumor types exist that may represent the oncogenic transformation of cerebellar precursor cells at different locations and stages. This scenario would be analogous to leukemias, in which oncogenic transformation of hematopoietic precursors at distinct developmental stages leads to distinct types of leukemias.
Cancer Stem Cells
Brain tumors have predominantly been classified as neuronal or glial in nature. The neuronal tumors include CNS PNETs, pineoblastomas, and medulloblastomas, as well as ganglion cell tumors. Glial tumors include many different gliomas, such as juvenile PA, subependymal giant cell astrocytoma, other low-grade astrocytomas, pontine glioma, malignant astrocytoma (including glioblastoma multiforme), and tumors that resemble other glial cell types, such as oligodendroglioma and ependymoma. Although this classification schema remains useful, it appears that many brain tumors are generated by oncogenic mutations in neural stem–precursor cells, rather than more mature cell types. Furthermore although cancers traditionally have been viewed as clonal, increasing evidence indicates that this is not the case. Instead the concept of the existence of a subpopulation of cancer stem cells has developed—distinctive cells within the tumor that are uniquely capable of regenerating the cancer. Recent studies of brain tumors have identified cluster of differentiation (CD)-133–positive cells as radioresistant, slowly proliferating cancer stem cells that are particularly prevalent in high-grade tumors, such as glioblastoma multiforme. The ability of these cancer stem cells to survive surgical resection, radiation, and cytotoxic chemotherapy is a major reason for the difficulty in curing high-grade brain tumors.
Genetic and Signaling Pathways Implicated in Development and in Pediatric Tumors
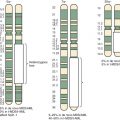
Inherited disorders that cause a familial propensity for brain tumors have provided an important method for identifying genetic pathways that contribute to these cancers. Neurofibromatosis, tuberous sclerosis, Gorlin syndrome, Turcot syndrome, Cowden syndrome, and the SMARCB1 (switch/sucrose nonfermentable [SWI/SNF]–related, matrix-associated, actin-dependent regulator of chromatin, subfamily b, member 1) mutation all represent heritable disorders associated with an increased risk of brain tumors ( Table 57-1 ).
| Chromosomal Abnormality | Tumor |
|---|---|
| Monosomy 22 | Atypical teratoid-rhabdoid tumor |
| Acoustic neuromas | |
| Meningioma | |
| Ependymoma | |
| 1p and/or 22q loss | Oligodendroglioma |
| Isochrome i17 | Medulloblastoma |
| 9q22 loss ( PTCH1 gene) | Medulloblastoma |
| Loss of chromosome 10, 9p, 17p | Progression to high-grade glioma |
Neurofibromatosis Type 1
Neurofibromatosis type 1 (NF-1) is an autosomal-dominant neuroectodermal disorder characterized by café-au-lait spots and fibromatous tumors of the skin. Additional clinical features that can be seen in NF-1 ( Box 57-1 ) include Lisch nodules in the iris, scoliosis, cognitive problems, and epilepsy. Several tumors occur with greater frequency in persons with this disorder, including pheochromocytoma, ependymoma, meningioma, and glioma; this characteristic may relate to dysregulation of specific stem cell populations. Among these tumors, gliomas of the optic pathway are the most common tumors seen. The unique biology of these tumors is slowly being elucidated through studies of the role of NF-1 in the development of the optic pathway in animal models. Neurofibromatosis is caused by heterozygous mutations in neurofibromin, and the cancers observed in this disorder result from loss of heterozygosity at chromosome 17q11.2, leaving only the mutant NF-1 allele. Although this disorder is inherited as an autosomal-dominant disease, as many as 50% of patients represent new germline mutations and therefore do not have a family history of the disorder.
* The diagnosis of neurofibromatosis type 1 requires any two or more of these criteria.
Six or more café-au-lait spots ≥1.5 cm in postpubescent individuals or >0.5 cm in prepubescent individuals
Two or more neurofibromas or one or more plexiform neurofibromas
Freckling in the axillae or groin
Optic glioma
Two or more Lisch nodules
Dysplasia of the sphenoid bone or dysplasia or thinning of the cortex of long bones
A first-degree relative with neurofibromatosis type 1
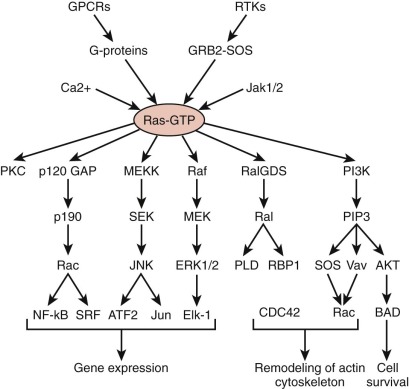
The neurofibromin protein is a 250-kD tumor suppressor that functions as a guanosine triphosphatase activator for the small G protein Ras. In this way, active neurofibromin decreases the ratio of guanosine triphosphate–bound (active) to guanosine diphosphate–bound (inactive) Ras or Ras-like protein. While activated Ras stimulates mitogen-activated protein kinases (MAPKs) and PI3Ks, the change in Ras activity leads to unregulated proliferation and survival ( Fig. 57-6 ). Although the incidence of brain tumors, particularly optic nerve gliomas, is significantly increased in persons with NF-1 (approximately 5% to 15%), the tumors that develop in these patients tend to be less aggressive than other gliomas. These tumors are more susceptible to chemotherapeutic interventions and thus can be treated differently than other gliomas. In fact many tumors stop growing spontaneously. The unique developmental environment of the optic pathways may account for the differential occurrence of these tumors in patients with NF-1, as well as their increased responsiveness. In addition to optic pathway gliomas (OPGs), non-OPGs occur at frequencies 100 times greater than expected, with the most common sites being the brainstem (49%), cerebral hemispheres (21%), and basal ganglia (14%). Other MRI signal abnormalities within the brain are often observed in patients with NF-1. The most characteristic abnormality is the unidentified bright object. Unlike low-grade gliomas (LGGs), these lesions are bright on T2-weighted imaging, do not demonstrate contrast enhancement, and usually produce neither mass effect nor symptoms. They often come and go and should not be biopsied or treated. The varied intracranial localization of lesions and variable need for neurosurgical intervention in a subset of children with NF-1 suggests that radiologic surveillance should be based on careful and regular neurologic and ophthalmologic examinations. Patients with NF-1 appear to be at increased risk of moyamoya syndrome, and this risk becomes especially high after cerebral radiation therapy. Patients with NF-1, even in the absence of a brain tumor, are also affected by a number of other problems as a result of their disease, in particular neurocognitive impairment, which can range from mild to severe.
Neurofibromatosis Type 2 (Merlin)
Neurofibromatosis type 2 (NF-2) is characterized by familial, bilateral acoustic neuromas and is caused by mutations in the gene that encodes merlin, or schwannomin, localized to chromosome 22q12.2. Merlin interacts with cytoskeletal components and appears to be important in adhesion-dependent growth control. Persons with germline mutations also have skin tumors with both peripheral schwannomas and neurofibromas and have a propensity to develop intracranial meningiomas or, more rarely, gliomas and spinal tumors. The onset of symptomatic tumor growth is uncommon in childhood, and in most patients the condition is identified in adulthood.
Tuberous Sclerosis
A third neurocutaneous disorder associated with an increased propensity for brain tumors is tuberous sclerosis (TS). This condition can be caused by mutations in either of two genes, TSC1 (hamartin, at chromosome 9q34) or TSC2 (tuberin, at chromosome 6p), and it is characterized by hamartomata in multiple organs. The most common clinical manifestations include epilepsy, cognitive and behavioral problems, and characteristic skin lesions. The white leaf-shaped skin lesions can best be seen under a Wood light; adenoma sebaceum (facial angiofibroma) can also be seen. Renal manifestations include angiomyolipomas, renal cysts, and, more rarely, renal cell cancer. Brain tumors develop in between 5% and 14% of patients, the most common being the subependymal giant cell astrocytoma (SEGA); other gliomas and ependymomas are also relatively frequent. Careful serial evaluations are required because of the possibility of additional tumor development in this patient population. Cortical tubers can cause seizures and require specialized neurosurgical approaches in children. Resection of tubers does not always control seizures and suggests that extratuberal epileptogenic brain abnormalities may be present that require more specialized imaging.
The phenotypic similarity of mutations in Tsc1 and Tsc2 is explained by the finding that these two proteins interact directly with one another. This complex acts as a guanosine triphosphatase–activating protein for Ras homolog enriched in brain (Rheb). The decreased activity of Rheb inhibits the mammalian target of rapamycin (mTOR) and p70 ribosomal S6 kinase–1. As a result there is diminished translation by eukaryotic translation initiation factor 4E-binding protein–1 (EIF4EBP1; 602223). The hamartin-tuberin complex thereby regulates growth and proliferation of subependymal and subventricular neural stem cells. The Tsc-mTOR pathways may normally be regulated by Wnt and insulin-like growth factor (IGF) ligands during development. Patients with TS who have SEGA or LGGs have demonstrated responses to mTOR inhibitors, confirming the clinical relevance of these findings.
Gorlin Syndrome
Gorlin syndrome (also known as basal cell nevus syndrome or nevoid basal cell carcinoma syndrome) is characterized by multiple basal cell carcinomas or basal cell nevi before the age of 30 years, odontogenic keratocysts or polyostotic bone cysts, and palmar and plantar pits. Other manifestations include rib or vertebral anomalies, large head circumference with frontal bossing, cardiac or ovarian fibroma, and lymphomesenteric cysts. Gorlin syndrome is caused by mutations in PTCH1 (chromosome 9q22.3), the receptor for the SHH ligand. Medulloblastoma develops in approximately 4% to 10% of persons with Gorlin syndrome. This syndrome also predisposes to other tumors, such as rhabdomyosarcoma and meningioma.
The PTCH1 gene product functions as both a receptor and negative regulator of signaling initiated by SHH or the related ligands, Indian hedgehog and desert hedgehog. These ligands initiate an unusual and incompletely understood signaling pathway ( Fig. 57-7 ). When a hedgehog ligand binds to Ptc, this alters the activation state of smoothened, or Smo, a seven-transmembrane protein. Normally Ptc represses the activity of Smo; however, when a ligand binds to Ptc, this derepresses Smo activity. Active Smo translocates to a distinctive subcellular organelle known as a primary cilium. Here active Smo enables the dissociation of a signaling complex containing suppressor of fused homolog (SUFU) and Glioma-associated Oncogene Homolog (Gli) transcription factors. The dissociation of this complex results in the nuclear relocalization of Gli family members and increased expression of Gli family members, as well as the expression of v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog (MYCN), D-type cyclins, and the stem cell–associated chromatic complex component, B lymphoma Mo-MLV insertion region 1 homolog (Bmi-1). The active pathway thereby potentiates proliferation and inhibits apoptosis.
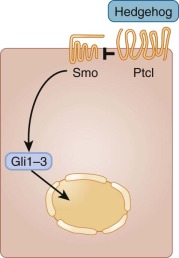
Good evidence indicates that constitutive activity of the SHH pathway can cause medulloblastoma. Gorlin syndrome is associated with increased incidence of medulloblastoma, as is the analogous mutation in mice. Activating mutations in Smo, or mutations in suppression of fused (SuFu), can also lead to these brain tumors. Recent studies have suggested that specific inhibitors of Smo may provide valuable biologic therapies for medulloblastoma.
The value of understanding developmental pathways that normally regulate neural precursor proliferation to decipher the mechanisms that cause pediatric brain tumors is reinforced by data showing that SHH ligand stimulates and regulates proliferation of granule cell precursors and neural stem cells. Thus it is perhaps not surprising that SHH-responsive genes are also expressed in gliomas, including diffuse intrinsic pontine gliomas (DIPG) and glioblastoma. Thus inhibitors of SHH signaling may play a role in treating several types of brain tumors.
Turcot Syndrome
Turcot syndrome is characterized by familial polyposis of the colon, together with malignant brain tumors. This disorder can be caused by mutations in the adenomatous polyposis coli gene ( APC , on chromosome 5q21) or in the mismatch repair genes MLH1 (120436) or PMS2 (600259). The distinction between the clinical entities that result from mutations in APC and mutations in repair genes include the nature of the brain tumors seen; the characteristic brain tumors seen are medulloblastomas or astrocytomas, respectively. APC is a large protein whose activity is critical in the Wnt signaling pathway. Therefore inactivating mutations of APC result in the aberrant accumulation of β-catenin and increased transcription of transcription factor 4 (Tcf4)-dependent genes, including c-myc . Mutations in β-catenin and in APC have also been reported in sporadic medulloblastoma, highlighting the importance of this pathway for this malignant cerebellar tumor.
Wnts constitute a family of ligands that can act through two distinct signaling pathways. The canonical Wnt pathway is initiated when Wnt proteins bind to cell-surface receptors of the Frizzled family. This binding leads to activation of Dishevelled (DSH) family proteins. When DSH becomes activated, it inhibits a protein complex that includes axin, glycogen synthase kinase–3 (GSK3), and APC ( Fig. 57-8 ). The axin–GSK3–APC complex promotes the proteolytic degradation of β-catenin. After this β-catenin destruction complex is inhibited, cytoplasmic β-catenin becomes stabilized and β-catenin is then able to enter the nucleus. Nuclear β-catenin interacts with TCF-LEF (lymphoid enhancer-binding factor 1) family transcription factors to promote the expression of a gene program that includes c-myc , N-myc , and cyclin D1 and thereby stimulate cell cycle progression ( Fig. 57-9 ). It is not clear whether the noncanonical Wnt pathway, which does not involve APC, also contributes to brain tumors.
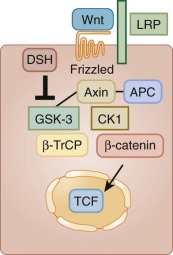
As is the case for SHH signaling, the ability of the deregulated Wnt pathway to cause medulloblastomas highlights the relevance of understanding neurodevelopment. In the absence of Wnt1, the cerebellum does not form properly. The Wnt and SHH pathways cooperate during normal development to generate normal cerebellar neurons. It is likely that these pathways also synergize in tumor formation, particularly during medulloblastoma oncogenesis.
Lhermitte-Duclos Disease, Cowden Syndrome, and PTEN Mutation
Activation of PI3K results in phosphorylation of phospholipids at the 3′ position; this phosphorylation is removed by the phosphatase and tensin homolog (PTEN) phosphatase. Therefore mutations in PTEN result in excess and/or incorrectly localized activation of the PI3K pathway. PI3K is critical in several signaling pathways that regulate proliferation, survival, migration, and cell size. The multiplicity of functions explains the diverse spectrum of disorders seen in PTEN mutations, including neurologic, cutaneous, and oncologic syndromes.
Molecular studies on Lhermitte-Duclos disease (LDD) tissue have revealed PTEN gene mutations in 83% of cases, with immunostaining showing lost or reduced PTEN expression in 78% of cases. As a consequence, Akt phosphorylation is increased. Initially Cowden syndrome was described as a familial predisposition for breast cancers, thyroid cancers, brain tumors, and other neoplasia. This syndrome was subsequently recognized as a spectrum of disorders that includes LDD and Bannayan-Ruvalcaba-Riley syndrome. The diagnosis of LDD depends on characteristic hamartomas of the cerebellum. These lesions in the cerebellar cortex exhibit thickened cerebellar folia, with misplaced cerebellar granule cells and enlarged size of the cerebellar neuronal cell bodies. In addition to cerebellar ataxia, these hamartomas can cause hydrocephalus and herniation. Another manifestation of the PTEN mutations is seen in Bannayan-Ruvalcaba-Riley syndrome, with macrocephaly, seizures, cognitive dysfunction, and autistic behaviors. Different manifestations of the mutation can be observed even within a family, and thus a careful consideration of family history is warranted.
RB1 Mutations
The retinoblastoma gene was the first tumor suppressor gene identified. In addition to the retinal tumors seen in persons with germline mutations in Rb1 , “trilateral” retinoblastoma has been described. In these persons, bilateral retinoblastoma is accompanied by a pineal tumor (pineoblastoma) with similar characteristics to retinoblastoma. The other secondary tumors that occur in patients with retinoblastoma are osteosarcomas. The Rb1 protein is required for the G1 checkpoint, and studies on this pathway have provided key insights into the mechanisms of growth regulation.
Atypical Teratoid Rhabdoid Tumor
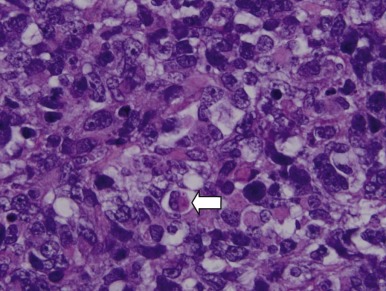
Mutations in the sucrose nonfermentable 5/integrase interactor 1 (SNF5/INI1) component of the SWI-SNF DNA remodeling complex cause rhabdoid tumors. These tumors include renal and soft tissue tumors and brain tumors. In the CNS, these brain tumors, called atypical teratoid rhabdoid tumors (ATRTs), are characteristically found in the cerebellopontine angle or in supratentorial locations. Prior to the identification of deletions or mutations of the SMARCB1 gene on chromosome 22, these tumors were historically grouped with medulloblastomas and PNETs. However their histology is distinct, with a mixture of atypical spindle cells, poorly differentiated small round blue cells, and rhabdoid cells with prominent cytoplasmic inclusions, large eccentric vesicular nuclei, and adjacent whorls of intermediate filaments. The nature of the cell of origin for these tumors, and why they predominantly arise in very young children, is as yet poorly understood.
Conceptual Organization of Pediatric Brain Tumors
Leukemias are considered in the context of their lineage and stage of development, whereas neuroblastoma is evaluated by the extent of spread, age of the child, and molecular phenotype. Neither of these approaches is well suited to CNS tumors. Although brain tumors share an anatomic site, a number of unique cell types, significant heterogeneity in distribution, and differences in the consequence of therapy differ according to the age of the patient and location within the CNS. These factors, combined with a complicated historic nomenclature, require a different approach to understanding these tumors.
The CNS is made up of three major elements, and therefore three major groups of tumors are commonly observed:
- 1.
Glial cells—responsible for structural support and maintenance of the CNS, and composed of three cell subtypes:
- a.
Astrocytes—structural support for the CNS → astrocytoma
- b.
Ependymocytes—help regulate homeostasis of the CNS → ependymoma
- c.
Oligodendrocytes—myelination for the neural axons → oligodendroglioma
- a.
- 2.
Neurons—electrical activity → medulloblastoma, pineoblastoma, CNS PNETs
- 3.
Choroid plexus—production of cerebrospinal fluid (CSF) → choroid plexus carcinoma (CPC)
Tumors arising from glia, neurons, or the choroid plexus account for approximately 90% of all pediatric CNS tumors. The remaining 10% of pediatric brain tumors arise from cells that are derived from extracranial sources but become entrapped in the developing CNS during embryogenesis ( Fig. 57-10 ).
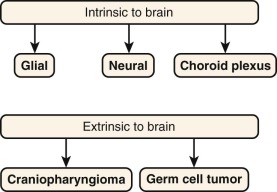
Figure 57-10
Most pediatric brain tumors can be classified into one of five different categories, based on cell of origin. Glial, neuronal, and choroid plexus tumors account for those that derive from cells within the central nervous system (CNS), while germ cell tumors and craniopharyngiomas arise from cells that are enclosed in the developing CNS in early development as a result of abnormal migration.
- 4.
Germ cells, which arise in the primordial gonadal ridge and normally migrate down to their final resting place in the abdomen (ovaries) or scrotum (testes), can occasionally migrate upward and become enveloped in the developing brain → germinoma, nongerminomatous germ cell tumor (NGGCT)
- 5.
Cells from the Rathke pouch, which normally gives rise to structures of the head and neck, can become trapped within the developing brain → craniopharyngioma
Two additional tumor types that are rare in children but account for approximately 80% of CNS tumors in adults include metastatic carcinoma, especially of the breast, colon, lung, and prostate. Metastatic lesions to the brain in pediatric patients are exceptionally rare, and when they occur, they are usually in the context of end-stage disease. Meningiomas are the other common adult brain tumor that is rarely observed in children.
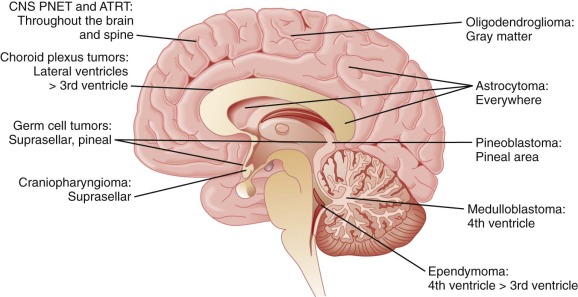
The five primary cell types of the brain are not evenly distributed in the CNS ( Fig. 57-11 ):
- 1.
Glia.
- a.
Astrocytes are found throughout the entire brain and spine.
- b.
Ependymocytes line each of the ventricles, and hence these cells are most predominant in the 4th ventricle > 3rd ventricle.
- c.
Oligodendrocytes are found around the junction of the gray-white matter.
- a.
- 2.
Neural tumors are defined by location rather than histology; molecular characterization indicates that these distinctions based on location are biologically important.
- a.
Medulloblastoma is found within the posterior fossa.
- b.
Pineoblastoma is found within the pineal region.
- c.
CNS PNETs can be found anywhere in the brain or spine.
- a.
- 3.
Choroid plexus is predominantly located in the lateral ventricles.
- 4.
Germ cell tumors (GCTs) are localized to the suprasellar region, the pineal area, or both.
- 5.
Craniopharyngiomas are found within the suprasellar region.
Brain tumors tend to spread in one of two ways, by direct invasion into adjacent regions with focal expansion of the primary mass, or by dissemination (seeding) of cells through the CSF, with resultant multifocal disease. Of the five tumor types listed, glial tumors and craniopharyngiomas tend to grow by direct extension and the other three tend to grow by seeding cells into the CSF. The workup of patients with newly diagnosed brain tumors will therefore require MRI of the involved area for glial tumors and craniopharyngioma, and craniospinal imaging and CSF cytology will be needed for seeding tumors (neural, choroid plexus, and germ cell).
Three major treatment strategies are considered for all CNS tumors: (1) surgery, (2) radiotherapy, and (3) chemotherapy. Certain general principles can be applied to their use ( Table 57-2 ):
- 1.
Surgery is important for making the diagnosis, achieving rapid reduction in tumor size, and relieving elevated pressure from obstructive hydrocephalus.
- 2.
Radiotherapy is effective for a wide range of tumors but has significant morbidity on the developing CNS, which frequently limits its applicability. In general focal tumors (gliomas and craniopharyngiomas) are treated with focal radiation therapy, whereas seeding tumors (medulloblastomas, pineoblastomas, CNS PNETs, CPCs, and GCTs) are treated with craniospinal radiotherapy with a boost to the primary site and areas of metastatic disease, particularly in children 3 years and older.
- 3.
Chemotherapy is effective for most seeding tumors and has become part of the initial therapy for these tumors (neural, choroid plexus, and germ cell). By contrast chemotherapy has had limited success for most focal tumors (malignant gliomas and craniopharyngiomas).
| Type of Tumor | Method of Spread | Attempted Surgery | Type of Radiation Therapy † | Chemotherapy |
|---|---|---|---|---|
| Glial | Local | Yes | Focal | No ‡ |
| Neuronal | Seeding | Yes | CSI | Yes |
| Choroid | Seeding | Yes | CSI | Yes |
| Germ cell | Seeding | Yes | CSI | Yes |
| Cranio | Local | Yes | Focal | No |
* Tumors that exhibit focal growth receive attempted resection and focal radiation therapy. Tumors that are at high risk for early dissemination are treated with focal surgery, CSI, and chemotherapy.
† Radiation therapy is often deferred in children younger than 3 years.
‡ Symptomatic unresectable low-grade gliomas are treated with chemotherapy to delay radiation therapy.
The classification of tumors based on their histologic characteristics is important to provide prognostic information, although the unique environment of the brain makes the classification of benign versus malignant less important than for most other sites in the body. The brain and spine are critical for the control of basic autonomic response, as well as higher order function, and therefore limit the ability to obtain complete resection with a wide margin in most cases. Even benign tumors located in critical and inoperable structures may result in death if their growth cannot be stopped or slowed. Conversely many highly malignant World Health Organization (WHO) grade IV brain tumors (defined histologically) that are responsive to radiation treatment (CNS germinoma) or radiation and chemotherapy (medulloblastoma) have an excellent prognosis. Because patients and their parents have preconceived notions about the importance of benign versus malignant, clarifying these terms early can be important.
The presenting symptoms for patients with CNS tumors can usually be categorized into one of two patterns: (1) direct compression of nerves or (2) obstructive hydrocephalus. The location of the tumor, histologic subtype, and age of the patient are major determinants in the length of clinical symptoms before the diagnosis is made. Although dependent on the location and rapidity of growth, the time to diagnosis for many children may range from 3 to 8 months, and multiple visits to primary care providers is not infrequent. For younger children who cannot verbalize their symptoms and for whom fine motor coordination, speech, and gait are still developing, even greater delays may result.
Symptoms related to the direct compression of adjacent nerves by a tumor will cause a unique constellation of symptoms that can be localized to an area as a result of the highly organized structure of the CNS.
- •
The posterior fossa contains the brainstem, 12 cranial nerves, and the descending and ascending fibers connecting the upper and lower aspects of the CNS, in addition to the cerebellum, which is responsible for movement and balance. Tumors in this area result in cranial nerve dysfunction such as diplopia, choking, or facial asymmetry. Tumors of the brainstem can compress the descending motor tracts, resulting in lower motor deficits. Compression of the cerebellum will lead to ataxia or dysmetria.
- •
The thalamus is the major relay station of coordinated function from the motor strip and other areas of the cortex. Tumors in this area will often lead to significant hemiparesis.
- •
The frontal lobe regulates mood and behavior and contains the motor cortex. Patients with tumors in this area will often present with changes in behavior (more aggressive or more passive), worsening school performance, or specific motor deficits (except those controlled by the cranial nerves). In some patients, more subtle signs of frontal lobe dysfunction such as fatigue, lack of interest, or decreased energy can be mistaken for the behaviors frequent in adolescence.
- •
The parietal lobe possesses the centers for sensory function. Tumors in this area can often compress a specific area of the sensory cortex, resulting in a focal sensory deficit that does not follow classic dermatomal or peripheral nerve patterns.
- •
The hypothalamus and suprasellar regions contain the area that coordinates endocrine function (i.e., growth hormone, regulation of salts, pubertal development, and stress hormones). This area is near the optic nerves and chiasm. Tumors in this area often present as a change in growth (accelerated or delayed), hormonal dysfunction, or change in vision.
- •
The occipital lobe organizes and interprets vision. Tumors in the occipital lobe will present with homonymous defects in vision.
- •
The pineal area sits adjacent to the centers of upward gaze (supranuclear tectal or pretectal areas). Lesions in this area can result in Parinaud syndrome (paresis of upward gaze, enlarged pupils that are poorly reactive to light, and poor or limited convergence).
- •
The spinal cord possesses all the ascending and descending tracks for sensory and motor function to all areas innervated from that segment of the cord and below. Mass lesions in this area will reduce the motor and/or sensory activity of those areas below the lesion and can consist of motor, sensory, temperature, position, and vibration abnormalities.
- •
Gray matter is where neuron bodies are concentrated. Lesions in the gray matter of the frontal, parietal, temporal, and occipital lobes can result in seizure activity. Although initially focal in nature, seizures can rapidly become generalized, obscuring the initial presenting focality.
- •
White matter tracts are the myelinated axons of neurons. Lesions in white matter tracts typically result in focal neurologic deficits that correspond to the tracts compressed.
The differential diagnosis of a new tumor of the CNS should be developed using the preceding information. Symptoms will help localize the probable site of the tumor. In turn the site will assist in developing a limited differential diagnosis of possible tumors at that site. Staging and treatment can then be considered in the context of focal versus seeding tumors. Although this exercise will not obviate the need for a definitive biopsy, it can help organize the large array of CNS tumors and ensure that appropriate presurgical planning and staging have been completed.
Obstructive Hydrocephalus and Raised Intracranial Pressure
The brain and spinal cord are supported in the cranium and spinal canal by the CSF, which is largely localized to the subarachnoid space. CSF is initially made by the choroid plexus in the lateral ventricles and, to a lesser degree, in the third and fourth ventricles. The production of CSF is not linked to its passage from the lateral and third ventricles to the fourth ventricle and, finally, through the foramen of Magendie or foramen of Luschka, where it is eventually reabsorbed by the arachnoid villi ( Fig. 57-12 ). The ventricles hold approximately 50 mL of CSF and, with approximately 500 mL of CSF produced each day, any failure to remove old CSF in the context of continued production will cause the fluid-filled ventricles to expand like water balloons in the closed cranium. Obstruction anywhere above the exit from the ventricles to the subarachnoid space (posterior fossa or above) will therefore result in obstructive hydrocephalus. The speed with which the accumulation of fluid occurs will in part determine the rapidity of the symptoms, as well as their severity. Obstructive hydrocephalus is considered a medical emergency because progressive expansion of the ventricular volume will force the brain to be compressed in all directions, including downward, resulting in tonsillar herniation.
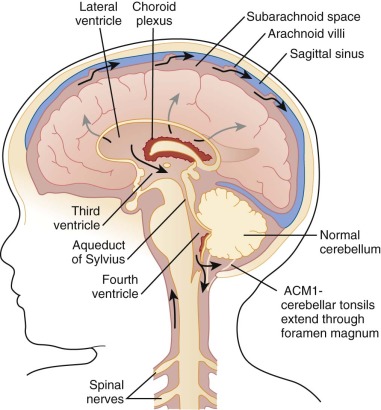
The three common symptoms of obstructive hydrocephalus include headaches, often severe in nature, that are thought to arise as a result of stretching of vessels and the pial surfaces. These headaches can often be worsened by changes in body position or head motion. They can be dull, aching, or stabbing in nature. Because of the prevalence of headaches in the general population, it is their persistence and worsening in the context of other symptoms (e.g., morning vomiting and focal neurologic deficits) that usually trigger further investigation. Patients will often have vomiting (often associated with a headache), especially early in the morning upon wakening. Whether this symptom results from hydrostatic pressure changes when first getting up, resulting in compression of the area postrema, or changes in CNS homeostasis upon wakening as a result of more rapid breathing and carbon dioxide release is unknown. Unfortunately the significance of morning vomiting is often overlooked and thought to be related to school avoidance or the flu. Many patients present with prolonged histories of intermittent morning vomiting and headache, suggesting that this process can be partial and relieved by the vomiting, which itself causes raised intraabdominal pressure and equilibration of the CSF pressure gradient. A third common symptom in children with hydrocephalus is blurring of the optic discs, related to the increase in intracranial pressure (ICP). Patients often report blurring or double vision, as well as difficulty in upward gaze. These symptoms likely result from a constellation of factors, including compression of the brainstem and cranial nerves, as well as edema and swelling of the optic discs (papilledema) and pathways leading to vision. The final common symptom of obstructive hydrocephalus is the presence of lower motor deficits, likely because of compression of motor tracts within the brainstem and difficulties with balance and gait related to pressure on the cerebellum.
The symptoms of obstructive hydrocephalus differ in infants, in whom the presence of open sutures permits the head to expand. This expansion relieves the buildup of pressure and thus the associated symptoms. While their head size expands, infants may begin to show some signs of delay in gaining milestones. Careful attention to head circumference changes will help identify these infants early, independent of the cause of the obstruction.
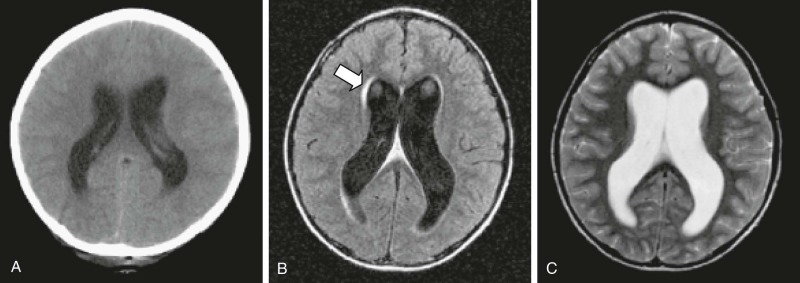
CT imaging of the brain is a rapid method for confirming the presence of obstructive hydrocephalus. Images (without the need for contrast material) will demonstrate enlargement of the ventricles above the area of obstruction. Increasing concerns of radiation dose regarding repeat CT imaging in young children has resulted in a shift to rapid-sequence MRI vent checks. These images are performed without contrast material and are not useful for assessing changes in the tumor. However they have the advantage of assessing changes in fluid within the CNS and without exposure of the child to ionizing radiation. MRI will show large ventricles on T1- and T2-weighted images. Best seen on fluid-attenuated inversion recovery (FLAIR) sequences, the presence of a bright signal around the ventricles is suggestive of transependymal flow, which is thought to result from the backward transduction of pressure from the ventricle to the brain parenchyma ( Fig. 57-13 ). Obstructive hydrocephalus is a surgical emergency and requires urgent intervention. For posterior fossa tumors such as medulloblastoma, ependymoma, or low-grade astrocytoma, relief from obstruction can be achieved with resection of the tumor in most patients, thus avoiding the need for a separate CSF diversion procedure.
Imaging Studies
Neuroimaging
Imaging of brain tumors entails determining the size and site of origin of the lesion, establishing primary diagnosis, and planning treatment. Neuroimaging is critical for the appropriate placement of catheters for stereotactic biopsy, resection, planning of radiation, guided application of experimental therapeutics, and delineation of tumor from functionally important neuronal tissue. After treatment, imaging is used to quantify response and the extent of residual tumor. At follow-up imaging helps determine tumor progression and differentiate recurrent tumor growth from treatment-induced tissue changes, such as radiation necrosis.
Imaging brain tumors in children presents unique challenges not encountered in adult imaging, including the need for sedation and consideration of the long-term effects on a growing child. Cranial CT and MRI remain the main modalities for the primary diagnosis of brain tumors. However several other techniques are being increasingly used in the evaluation of this patient population, including positron emission tomography (PET), MR perfusion and diffusion, and MR spectroscopy (MRS). Assessment of response in pediatrics is a critical component in the appropriate treatment of patients, as well as in identifying active regimens. Recently adult neurooncologists have adopted a new series of guidelines (developed by the International Radiologic Assessment in Neuro-Oncology Committee) to aid in better differentiating tumor progression from pseudoprogression and similarly tumor response from pseudoresponse. Although some of these adult criteria will be useful in pediatrics, the types of tumors common in the pediatric population will require some modifications to the adult approach. Currently an international working group is developing recommendations for children with brain tumors (Radiologic Assessment in Pediatric Neuro-Oncology).
CT is a rapid and inexpensive modality for assessing fluid, blood, and calcification in the central CNS. As such it has typically been the first imaging procedure in children with a presumed intracranial bleed or raised ICP that might require immediate neurosurgical intervention. Other than for lesions arising from the skull vault and to assess calcified tumors, CT scans are used less frequently for routine surveillance of pediatric patients because of an increase in long-term cancer risk caused by CT imaging.
MRI is currently the modality of choice for localization and assessment of the size of brain tumors. MRI provides valuable information about secondary phenomena such as mass effect, edema, hemorrhage, necrosis, and signs of increased ICP. In addition MRI provides excellent tissue contrast and high spatial resolution. Standard T1- and T2-weighted MRI sequences detect brain tumors with high sensitivity. Varying acquisition parameters such as T1 or T2 weighting, techniques such as diffusion- and perfusion-weighted images, and FLAIR sequences reveal a characteristic pattern of each tumor, depending on tumor type and grade. Susceptibility-weighted MRI is useful for detecting areas of hemorrhage, calcifications, and increased vascularity associated with brain tumors. Recently rapid-sequence MRI for the assessment of bleeds or hydrocephalus has been developed, which can avoid the radiation doses associated with CT scans and, like CT scans, can be performed without sedation, even in young children. However it should be noted that these studies are limited in evaluation of the tumor burden and should only be used to assess for acute findings.
MRS can also be helpful for the initial characterization of tumors ; it can also be used to differentiate tumor tissue from other tissue in children with CNS tumors in certain circumstances. MRS has continued to evolve, and the development of multivoxel and two-dimensional techniques has resulted in improved spatial resolution, thereby supplying additional information regarding tumor heterogeneity and intratumoral metabolite distribution. Although MRS is a sensitive technique, it still lacks specificity as a stand-alone technique in the clinical setting. In a recent study, brain proton MRS biomarkers were shown to predict survival of children with CNS tumors better than standard histopathology. More accurate prediction using this noninvasive technique represents an important advance and may suggest more appropriate therapy, especially when a diagnostic biopsy is not feasible. In general, decreased N -acetylaspartate (NAA) and creatine concentrations and increased choline concentrations correlate with tumor grade. Reduction of NAA is likely because of neuronal death or damage, although the reduction in creatine is likely to be a result of changes in cell energetics. The increase in choline is believed to reflect increased membrane synthesis. Increases in lipid and lactate concentrations have been observed in some gliomas. Lactate accumulation is believed to be a result of central tumor necrosis.
Diffusion-weighted MR pulse sequences enable a quantitative and reproducible assessment of the diffusion changes, not only in areas exhibiting signal abnormality in conventional MRI but also in areas of normal signal. MR diffusion using predominantly echoplanar techniques has been useful in the characterization of tissue, tumor cellularity, tumor grading, tumor response to treatment, and distinction of tissue types. Diffusion tensor imaging (DTI) provides visualization of fiber bundle direction and integrity, with in vivo characterization of the rate and direction of white matter diffusion. DTI is useful for presurgical planning or coregistration of tractography data with radiosurgical planning and functional MRI data. Fractional anisotropy using DTI may prove helpful for the assessment of treatment-induced white matter changes in children.
The usefulness of diffusion-weighted imaging (DWI) for characterizing intracranial cystic or cystlike lesions has been demonstrated in a number of studies. DWI has long been used to differentiate between epidermoid and arachnoid cysts. Arachnoid cysts are characterized by free diffusion, whereas epidermoids have an apparent diffusion coefficient similar to that of brain parenchyma, thereby demonstrating restricted diffusion. The usefulness of DWI in the distinction between ring-enhancing cerebral lesions such as brain abscesses, cystic or necrotic HGG, or metastasis has been shown in multiple studies during the past decade, although this differentiation continues to be a challenge. The ring enhancement of a brain abscess can be indistinguishable from that of a cystic or necrotic HGG or metastasis. Other lesions that may also have a similar appearance are subacute ischemic infarction, resorbing hematoma, and demyelinating disease. Abscesses demonstrate high signal on DWI and a reduced apparent diffusion coefficient (ADC) in a cystic ring-enhancing cerebral lesion. ADC values have been assessed between tumor types; however, considerable overlap exists between certain tumor types, requiring additional evaluations. Authors of a retrospective study of ADC values of 275 adult and pediatric brain tumors have reported a significant negative correlation between ADC and WHO astrocytic tumor grades II through IV. Other comparisons included a higher ADC in dysembryoplastic neuroepithelial tumors (DNETs) than in astrocytic grade II tumors (100% accuracy) or other glioneuronal tumors, a lower ADC in malignant lymphomas compared with glioblastomas and metastatic tumors, a lower ADC in CNS PNETs compared with ependymomas, and a lower ADC in meningiomas compared with schwannomas. The ADC of craniopharyngiomas was higher than that of pituitary adenomas, whereas the ADC of epidermoid tumors was lower than that of chordomas. In meningiomas the ADC was not indicative of malignant grade or histologic subtype. DWI has also been used to obtain additional information regarding tumor type and grade. The reduction in extracellular space, as well as high nuclear-to-cytoplasmic ratios of some cancer cells, causes a relative reduction in ADC values. In some studies overlap was seen in ADC values of HGGs and LGGs. The presence of glycosaminoglycans such as hyaluronan in the extracellular space of some HGGs may decrease water content and cause a reduction in ADC values. In addition, one pitfall of DWI is that high-grade tumors that may exhibit necrosis can lead to higher ADC values.
DWI and proton MRS have been evaluated as diagnostic tools, and in a study of children with posterior fossa lesions in which these techniques were combined, MRI was successful in correctly identifying the histologic diagnosis in every case. Although this approach does not replace the pathologic diagnosis, it demonstrates the increasing accuracy of biologic-based imaging. Similar results have been reported for ADC analysis. DWI may also be helpful in differentiating postsurgical changes from tumor recurrence. DWI can also detect acute changes in white matter from methotrexate administration, which must be differentiated from progressive disease.
Determination of the tumor margins is considered by many investigators to be extremely important for the management of brain tumors. Complete resection of tumors with minimal neurologic deficit is the ultimate goal of surgical resection. In some studies DWI has been shown to discriminate among tumor, infiltrating tumor, peritumoral edema, and normal brain parenchyma. However other studies have not found DWI to be helpful for evaluating tumor margins.
MR diffusion imaging has also been assessed as a biomarker for early prediction of treatment response in patients with brain tumors. Recent studies have indicated the possibility of using functional diffusion map analysis as an early biomarker for treatment response preceding decrease in tumor size. Increasingly MR perfusion imaging is being used to evaluate cerebral perfusion dynamics by analysis of the hemodynamic parameters of relative cerebral blood volume (CBV), regional cerebral blood flow (CBF), and mean transit time. CBV is the parameter most commonly quantified in brain tumors. CBV is defined as the volume of blood in a region of brain tissue, commonly measured in milliliters per 100 g of brain tissue. CBF refers to the volume of blood/unit time passing through a given region of brain tissue, measured in milliliters per minute per 100 g of brain tissue. Mean transit time refers to the average time it takes blood to pass through a given region of brain tissue and is commonly measured in seconds. Perfusion imaging techniques include T2-weighted dynamic susceptibility techniques, arterial spin labeling (ASL) techniques, and T1-weighted dynamic contrast-enhanced perfusion techniques. These techniques use exogenous tracer agents, such as paramagnetic contrast material, or endogenous tracer agents, such as magnetically labeled blood (arterial water). The most common method currently performed in the clinical setting is dynamic contrast-enhanced perfusion MRI with an exogenous tracer, such as gadopentetate dimeglumine. It is assumed that the tracer is restricted to the intravascular compartment and does not diffuse into the extracellular space. Imaging is performed dynamically (rapid imaging over time during a bolus injection) using echoplanar imaging–based spin echo or gradient echo sequences. It is thought that the spin echo sequences are more sensitive to capillary level blood vessels, whereas gradient echo techniques are more sensitive to the larger vessels. Although gradient echo sequences are associated with more magnetic susceptibility artifacts, particularly in the posterior fossa, they are the more common of the two techniques. For young children and infants, challenges exist relating to intravenous (IV) access, smaller intravenous catheters, and limitations of the contrast medium dose.
DTI is an adaptation of DWI and is performed by acquiring diffusion data in six or more directions, enabling determination of the direction and magnitude of water diffusion. Connecting the directions of diffusion in each voxel to those of neighboring voxels using a variety of mathematical algorithms enables creation of a three-dimensional (3D) white matter tract map, termed “tractography.” This technique is used to delineate important white matter tracts affected by tumor and help guide surgical resection. In conjunction with functional MRI, tractography can be used to predict possible postoperative deficits resulting from white matter tract damage.
Dynamic T1-weighted contrast imaging can be used to assess microvascular permeability (measured as the transendothelial transfer constant, or Kps) in brain tumors. Kinetic modeling of the dynamic signal changes can yield estimates of regional fractional blood volume and Kps, which is an indicator of BBB disruption and correlates with angiogenesis. This technique can be successfully performed in children, and applications of this technique may be useful for monitoring antiangiogenic therapies in pediatric patients with brain tumors. ASL is an MR perfusion technique that does not use an IV contrast agent. The perfusion contrast in the image results from the subtraction of two successively acquired images, one with and one without proximal labeling of arterial water spins, with a magnetic gradient used to invert the magnetization of inflowing blood. The signal-to-noise ratio, anatomic coverage, and shorter imaging time are currently better for the dynamic contrast perfusion techniques compared with ASL. However ASL may have a future role in the imaging of pediatric brain tumors, particularly because it relies on a noninvasive endogenous contrast agent.
The use of PET and single-photon emission CT (SPECT) imaging continues to improve and can be important in helping to differentiate treatment effect from tumor recurrence. The usefulness of PET imaging is especially evident when a baseline evaluation is performed so that postoperative changes can be evaluated in the context of the pretherapy PET avidity, thus requiring consideration of nuclear imaging early in the workup of these patients.
Standardization of neuroimaging parameters for children with CNS tumors and the testing of novel sequences that can be adapted to specific molecular inhibitors now being evaluated in this population are being developed.
A further advance in MRI of brain tumors has occurred with the availability of intraoperative scanners. These scanners enable preoperative guidance for stereotactic biopsy and for planning tumor resection, and they provide a review of the resection site for residual tumor prior to closure of the craniotomy. Intraoperative DTI has been proposed to aid in the preservation of fiber tracts and to minimize postoperative deficits.
Somatostatin receptor scintigraphy has been used to differentiate the presence of residual or recurrent tumor from scar and necrosis and is better than MRI scans for a number of pediatric tumor types. Molecular imaging is likely to play an expanding role in neurooncology as more pathway-specific inhibitors become available.
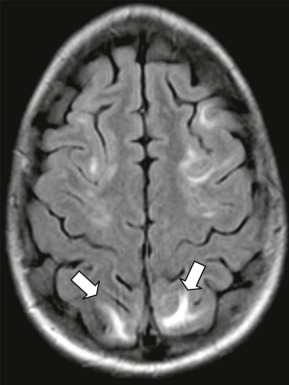
Posterior reversible leukoencephalopathy (PRES) is increasingly identified in children with brain tumors, particularly in children with episodes of hypertension. Patients present with headaches that are usually severe, mental and visual status changes, and seizures concurrent with hypertension and characteristic MRI findings, including T2 signal abnormalities. MRI findings are those of vasogenic edema with T2 and FLAIR hyperintensities involving predominantly the parietal and occipital regions bilaterally ( Fig. 57-14 ). The diffusion changes in PRES are traditionally thought to be represented by higher ADC values, consistent with vasogenic edema. Focal areas of restricted diffusion (likely representing infarction–tissue injury with cytotoxic edema) are uncommon (11% to 26%) and may be associated with an adverse outcome. Hemorrhage (focal hematoma, isolated sulcal-subarachnoid blood, or protein) is seen in approximately 15% of patients. The parietal and occipital lobes are most commonly affected, followed by the frontal lobes, the inferior temporal-occipital junction, and the cerebellum. Lesion confluence may develop as the extent of edema increases.
The mechanism of PRES remains controversial, although the hypertension-hyperperfusion theory is favored because of the common presence of elevated blood pressure and perceived response to hypertension management. Key issues remain problematic, including PRES in normotensive patients with pressures rarely reaching autoregulatory limits, and brain edema that is lower in patients with severe hypertension. Hypertensive encephalopathy animal models do not reflect the systemic toxicity that is present, and hyperperfusion has not conclusively been demonstrated in patients.
Intracranial vasospasm has been seen with conventional and MR angiography, suggesting vasospasm as a possible pathophysiologic mechanism for the observed findings. MR DWI was instrumental in establishing and consistently demonstrating that the areas of abnormality represent vasogenic edema. Prompt treatment with antihypertensive therapy or discontinuation of immunosuppressive agents can lead to complete recovery in some cases. However, if untreated, permanent neurologic deficits or even death may occur as a result of cerebral infarctions or hemorrhages, and 20% to 40% of patients with PRES can be normotensive. PRES can be associated with a number of inciting events, including chemotherapy, radiation therapy, and antiangiogenic drugs. This latter group of drugs may cause PRES as a result of their direct effect on vascular endothelial growth factor (VEGF) and raised blood pressure. Rapid recognition of this entity is critical to prevent permanent damage from occurring.
Surveillance Imaging
The role and usefulness of surveillance imaging for patients with a brain tumor remain controversial and depend on a number of factors, such as the age of the patient, histology of the tumor, time from diagnosis, and type of treatment. For example, in one study only nine of 318 imaging encounters identified an asymptomatic recurrence. Other studies have demonstrated the cost-effectiveness of surveillance imaging, recognizing that decisions are often made on the basis of insurance coverage. A common practice has been imaging every 3 months while the patient is undergoing therapy (to assess continued response while undergoing therapy), and then every 3 months for the first year after the completion of therapy. Beginning in the second year, scans are performed every 6 months for a year and then annually afterward. With time the risk of tumor recurrence will go down, although the risks of radiation-induced vasculopathy and second tumors begin to increase. Modification of these guidelines for children with tumors at low risk of recurrence (e.g., completely resected craniopharyngioma or low-grade astrocytoma) or those who did not receive radiation therapy can be made on a case-by-case basis.
Neuropathology
The neuropathologic classification of pediatric brain tumors has evolved greatly during the past century. Categorizing tumors is helpful to guide therapy and estimate prognosis. A number of outstanding reviews on the classification of CNS tumors have been written. When attempting to determine the treatment and/or prognosis of a tumor based on published reports or meeting abstracts, the classification schema used in those reports will become critical before applying this information to other patients.
Most current classification systems are based on the pioneering work of Cushing and Bailey almost 100 years ago. The major premise of this approach was to define tumors by their presumed cell of origin and cell lineage based on morphologic similarity to normal immature or mature brain cells. This system was adapted by Kernohan when he proposed that certain tumors, especially those with a glial appearance, such as astrocytomas, ependymomas, and oligodendrogliomas, could be further classified by the degree of anaplasia, which related to prognosis. Most current systems now use these two criteria—presumed cell lineage and degree of anaplasia—as the primary basis for classification of adult and pediatric CNS tumors. In spite of the usefulness of this classification schema, it is becoming progressively clear that most brain tumors do not derive from mature cell types but rather from primitive precursors or stem cells that can differentiate down many different pathways, obscuring the cell lineage. In comparison with their adult counterparts, pediatric brain tumors are exceptionally diverse in their morphologic appearance and therefore represent a particular challenge for classification by morphologic criteria alone. This diversity likely stems from the fact that pediatric brain tumors are derived from a wide spectrum of proliferative cell types at many developmental stages not present in adult brains.
Significant advances have been made in the field of neuropathology. The WHO classification of CNS tumors has been recently revised, with a number of important modifications based on histopathologic recognition of new pediatric brain tumors. A synopsis of the WHO 2007 classification system is provided in Table 57-3 . Increasingly the field has begun to include use of molecular alterations for many tumors and the use of highly specific immunohistochemical markers. When combined with immunohistochemical analysis and the increasing use of cytogenetic classification and molecular profiling, the classification of tumors continues to become more reproducible and predictive of clinical outcomes. A simplified overview of the chromosomal abnormalities associated with pediatric brain tumors is presented in Table 57-1 . For many large consortium-based studies in both Europe and North America, molecular profiling of tumors to ensure proper classification is now required, especially for medulloblastoma and ATRTs.
| Type | Subtype | Example(s) | Grade |
|---|---|---|---|
| Glial tumors | Astrocytic tumors | Pilocytic astrocytoma | I |
| Subependymal giant cell astrocytoma | I | ||
| Pilomyxoid astrocytoma | II | ||
| Pleomorphic xanthroastrocytoma | II | ||
| Diffuse (fibrillary) astrocytoma | II | ||
| Anaplastic astrocytoma | III | ||
| Glioblastoma multiforme | IV | ||
| Gliosarcoma | IV | ||
| Gliomatosis cerebri | III-IV | ||
| Oligodendroglial tumors | Oligodendroglioma | II | |
| Oligoastrocytoma | II | ||
| Anaplastic oligodendroglioma | III | ||
| Anaplastic oligoastrocytoma | III | ||
| Ependymal tumors | Subependymoma | I | |
| Myxopapillary ependymoma | I | ||
| Ependymoma | II | ||
| Anaplastic ependymoma | III | ||
| Neural/embryonal tumors | Medulloblastoma | IV | |
| Pineocytoma | I | ||
| Pineal parenchymal tumor of intermediate differentiation | II-III | ||
| Papillary tumor of the pineal region | II-III | ||
| Pineoblastoma | IV | ||
| Primitive neuroectodermal tumor (medulloepithelioma, ependymoblastoma) | IV | ||
| Atypical teratoid-rhabdoid tumor | IV | ||
| Choroid plexus tumors | Choroid plexus papilloma | I | |
| Atypical choroid plexus papilloma | II | ||
| Choroid plexus carcinoma | III | ||
| Germ cell tumors | Germinoma | III | |
| Nongerminoma | Embryonal carcinoma | III | |
| Yolk sac tumor | III | ||
| Choriocarcinoma | III | ||
| Mature teratoma | 0 | ||
| Teratoma | I | ||
| Immature teratoma | III | ||
| Teratoma with malignant transformation | III | ||
| Craniopharyngioma | Craniopharyngioma | Adamantinomatous | I |
| Papillary | I | ||
| Other | Mixed glial neuronal tumor | Ganglioglioma | I |
| Gangliocytoma | I | ||
| Anaplastic ganglioglioma | III | ||
| Dysembryoplastic neuroepithelial tumor | I | ||
| Desmoplastic infantile astrocytoma | I | ||
| Central neurocytoma | II | ||
| Extraventricular neurocytoma | II | ||
| Neuroepithelial | Astroblastoma | — | |
| Nerve tumors | Schwannoma | I | |
| Neurofibroma | I | ||
| Malignant peripheral nerve sheath tumor | II-IV | ||
| Meningeal | Meningioma | I | |
| Atypical meningioma | II | ||
| Anaplastic meningioma | III | ||
| Hemangioblastoma | I |
The immunohistochemical patterns used to classify CNS tumors require considerable experience on the part of the neuropathologist, as well as appropriate control subjects. Most markers lack specificity and can be identified in a wide array of histologies, requiring correlation with other clinical or molecular data. Common markers used to classify pediatric CNS tumors are provided in Table 57-4 . Four commonly used immunohistochemical markers are the oligodendrocyte lineage transcription factor 2 (OLIG2) and glial acidic fibrillary protein (GFAP), which stain glial cells ( Fig. 57-15 ); synaptophysin, which stains neurons ( Fig. 57-16 ); and Ki-67, which stains cells that have left G0 cell cycle and are at some stage of cellular division ( Fig. 57-17 ).
| Marker | Tumor |
|---|---|
| Glial fibrillary acidic protein | Astrocytoma, oligodendroglioma, ependymoma, choroid plexus papilloma, PNET, ATRT |
| Synaptophysin/NeuN | PNETs, ganglial tumor, neurocytoma |
| MIB1/Ki-67 | Measures all cells not in G0 |
| Mitotic rate | Measures cells in mitosis |
| Neurofilament proteins | Ganglial tumor, PNET, neurocytoma, subependymal giant cell tumor, ATRT |
| S-100 and neuron-specific enolase | Normal and neoplastic glial and neuronal in origin |
| Retinal S-antigen | Pineal parenchymal tumor, PNET, retinoblastoma |
| Desmin | Muscle tumor, teratoma, PNET |
| Smooth muscle actin | Muscle tumor, ATRT |
| Cytokeratin | Chordoma, choroid plexus tumor, meningioma, some malignant gliomas, nongerminomatous germ cell tumor, PNET, ATRT |
| Epithelial membrane antigen | Meningioma, ependymoma, teratoma, ATRT |
| Vimentin | Mesenchymal tumor, meningioma, sarcoma, melanoma, ependymoma, astrocytoma, chordoma, schwannoma, PNET, ATRT |
| Alpha-fetoprotein | Embryonal carcinoma, endodermal sinus (yolk sac) tumor |
| Human chorionic gonadotropin | Germinoma, choriocarcinoma |
| Placental alkaline phosphatase | Germ cell tumor |
| INI-1 | ATRT |
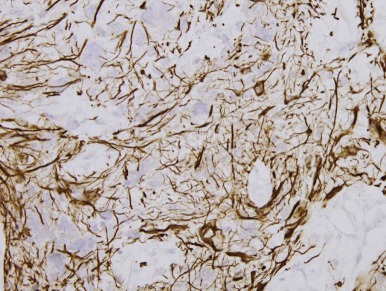
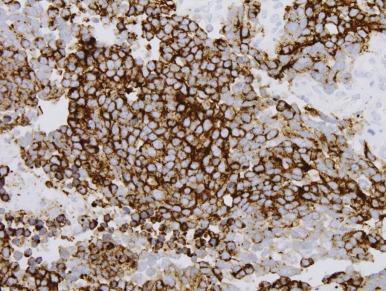
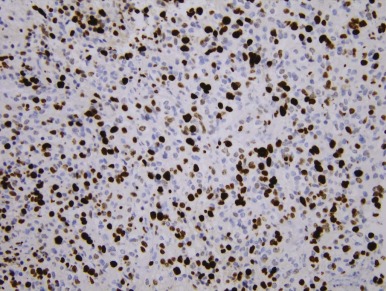
Treatment Strategies
Neurosurgery
The neurosurgeon is often the first of the specialized team of caregivers called to see a child with a brain tumor, with the call frequently coming from the emergency department or radiology department. The initial evaluation consists of acute management—that is, assessment of the patient’s clinical condition with respect to neurologic stability and the possibility of acute neurologic decompensation requiring immediate intervention, and subsequent to that the development of a plan that includes surgical interventions geared toward obtaining tissue for pathologic diagnosis, resection of tumor as part of the overall management of the tumor, and dealing with the secondary effects of the tumor.
In the field of neurosurgery remarkable advances have occurred in technology, from intraoperative microscopes, robotics, and computer-assisted navigation to intraoperative MRI, endoscopic techniques, and minimally invasive techniques. Each of these advances has been instrumental in reducing the morbidity of neurosurgical procedures while ensuring maximal surgical resections.
Acute Management Issues
Hydrocephalus.
Hydrocephalus is the most common clinical presentation of posterior fossa brain tumors, which are the most common pediatric brain tumors. Historically the initial evaluation of children suspected of harboring a posterior fossa brain tumor has been via CT scan. However with improved technologies and more recent concerns regarding the long-term effects of radiation exposure, there is a trend toward less utilization of CT scans. If possible the initial assessment should be an MRI of the brain, which can provide the dual information regarding the brain tumor and the extent of hydrocephalus. The cause of the hydrocephalus is obstruction to the CSF flow as a result of the tumors in and around the fourth ventricle. The evaluation should consist of assessing the severity of the hydrocephalus (via the presence of periventricular capping/edema and tonsillar herniation) and the need for immediate intervention. High-dose steroids will often produce significant relief in symptoms as peritumoral edema is suppressed, and they are often utilized as a temporary measure for symptom relief. In infants, hydrocephalus can be asymptomatic and only evidenced by a rapidly enlarging head circumference. Supratentorial brain tumors can also produce hydrocephalus by causing mass effect on the ventricles, as well as by obstructing CSF flow through the ventricular system. Patients with suprasellar tumors can also have inadequate cortisol production or electrolyte disturbances, which need to be addressed as quickly as possible and often in concert with preparation for surgery.
Raised ICP is a medical emergency. Immediate relief can be accomplished by temporary placement of a catheter (drainage tube) in the ventricles, although this step is rarely needed, primarily because of the fact that steroids in the preoperative period followed by surgery to remove the tumors within 24 to 48 hours after diagnosis usually relieves the obstruction. Permanent techniques of hydrocephalus treatment include the placement of a ventriculoperitoneal (VP) shunt that drains CSF around the obstruction into the peritoneal space via a permanently implanted tube and more recently via an endoscopic third ventriculostomy (ETV). The latter has become the treatment of choice in the management of obstructive hydrocephalus and is not associated with the long-term complications of shunt revisions and infections. Most pediatric centers with sufficient expertise now routinely perform ETVs as the primary management of hydrocephalus in children with brain tumors. In addition the overall incidence of shunting for brain tumors has decreased during the past two decades. Like all complex procedures, rare but significant neurologic risks are associated with this procedure in approximately 1% of cases. The possibility of closure of a third ventriculostomy and resultant acute hydrocephalus requires long-term follow-up with neurosurgery, as with all children who have had placement of shunts for the management of the hydrocephalus.
Visual and Endocrine Evaluation.
Children with tumors in the sellar and suprasellar area can present with blindness, which rarely can be acute in onset. In those circumstances, although extremely rare, it is possible to obtain some recovery of vision by immediate treatment of the tumor. If a large mass or cyst is causing compression, acute drainage in conjunction with steroid administration can lead to some recovery of vision. Tumors in this location can also interfere with endocrine function and may lead to electrolyte disturbances and decreased steroid production, and these effects can be critical if they are unrecognized, especially if acute surgical intervention is indicated.
Surgical Management
Surgical management after treatment of hydrocephalus consists of obtaining tissue for diagnosis and resecting the tumor. The decision to perform these procedures depends on the location of the tumor, the known biology, and the goals of treatment management.
Certain tumors do not require routine biopsy or attempted resection at diagnosis (e.g., diffuse pontine gliomas, classic optic pathway tumors in children with NF-1, and some suprasellar or pineal lesions with positive serum or CSF tumor markers). Tectal gliomas are rare tumors centered in the tectum with a classic radiographic appearance and presentation of hydrocephalus and, rarely, neurologic deficits. Their management consists solely of treatment of the hydrocephalus via ETV and radiographic and clinical follow-up.
The leading factor in the outcome of a child with a brain tumor is not the presenting symptoms, age of the child, or the final disease. Rather it is the experience of the neurosurgeon performing the operation and the number of similar procedures performed during the prior few years. With increasing cure rates in children with CNS tumors, careful consideration of the neurosurgical morbidity, which can often be lifelong, needs to be taken into account while a treatment plan is developed.
Advances in Surgical Techniques.
Recent advances in image-guided neurosurgical techniques have been considerable. Endoscopic procedures provide minimally invasive techniques to address not just obstructive hydrocephalus but also biopsy or resection of intracranial masses, with minimal morbidity in experienced hands. New techniques using intraoperative ultrasound have been developed and are being used in pediatric patients. 3D laser-guided maps can assist in the orientation and surrounding environment of the tumor during a procedure. More recently intraoperative MRI facilities now allow surgeons to use MRI procedures with the same magnet strength as that used for diagnostic imaging while operating. Real-time MRIs can assist the neurosurgeon in the detection of residual disease or hemorrhage before closure of the resection site.
The surgical approach used by the neurosurgeon will depend on a number of factors that must balance the need for diagnosis with the potential risks of operating in a given area. While the relative risks of general anesthesia and neurosurgical procedures continue to diminish with improving techniques, it is now common to perform staged operations in which a limited resection is performed to confirm the diagnosis. Based on the pathologic results obtained, decisions about more complicated or risky surgery can be considered for lesions in which complete resection is a critical component of improved outcome and can be deferred in lesions in which additional resection would not significantly alter prognosis but could cause significant morbidity. Specialized techniques such as endonasal endoscopic approaches can allow a minimally invasive approach, with excellent outcome in centers that have expertise in this approach.
The need of neurosurgeons to be aware of the evolving treatment strategies for children reinforces the role of the multidisciplinary team. During the past 5 years this need has become even more important, because many pediatric brain tumors now require molecular classification to guide therapy. For example in spite of their similar appearance, the treatment of posterior fossa medulloblastoma now differs significantly from posterior fossa ATRT. Treatment on national protocols therefore requires submission of fresh-frozen material obtained at the time of surgery for proper stratification on the basis of therapy protocols. As our molecular classification of pediatric brain tumors expands and newer and better molecular inhibitors of specific pathways become available, the role of the neurosurgeon and neuropathologist in ensuring the proper processing of these samples will continue to expand.
Perioperative Issues.
A number of factors must be considered for a child after a tumor-based procedure has been completed. The rate of weaning steroids after an operation will depend on many factors, including the histology, degree of resection, postsurgical edema, and patient status. Although most patients can be weaned off steroids rapidly after an operation, it is important that the members of the team have a coordinated structure so that as children pass from neurosurgical care to the radiation therapist or oncologist, overall management of these issues is seamless. A similar discussion holds true for anticonvulsant agents. Whereas many patients will receive preoperative or perioperative anticonvulsant therapy, most can be weaned rapidly, and communication among team members will therefore be required.
Seizures can be a presenting symptom for many patients with brain tumors or a result of electrolyte disturbances in the perioperative period. Management includes anticonvulsant agents and surgical resection geared not only toward the tumor but also toward dealing with the seizures. Intraoperative electrocorticography is particularly useful in the management of temporal and frontal lobe tumors with seizures and requires a multidisciplinary approach, which can lead to excellent overall outcomes. Choices of antiepileptic drugs (AEDs) are important because some may interfere with metabolism of chemotherapeutic agents, which requires a comprehensive approach toward the management of the patient’s seizures and tumor.
Hyponatremia is a common problem that can occur after neurosurgical intervention and requires immediate recognition. Two common conditions, both resulting in hyponatremia but needing different interventions, can occur. Cerebral salt wasting (CSW) occurs because of excess renal loss of sodium with volume depletion and has been associated with abnormally high atrial natriuretic peptide or brain natriuretic peptide levels, which block all stimulators of zona glomerulosa steroidogenesis, resulting in mineralocorticoid deficiency. CSW usually occurs 1 to 2 days after neurosurgical intervention, and patients typically demonstrate polyuria, dehydration, a serum sodium level less than 130 mEq/L, and excess urine sodium or urine osmolarity. Duration of CSW was 1 to 9 days in a study of 12 pediatric patients. CSW is generally treated with salt repletion, although fludrocortisone supplementation has also been successful. Syndrome of inappropriate diuretic hormone (SIADH), by contrast, results when water is preferentially retained, causing dilution of serum sodium. Patients present with hyponatremia and an elevated urine sodium level. The major clinical difference between CSW and SIADH is that in the former, dehydration is common, whereas in SIADH, symptoms of dehydration are lacking. SIADH is treated with water restriction. CSW was much more common than SIADH in one study of 30 patients.
Posterior fossa syndrome (or cerebellar mutism syndrome) is a complex and heterogeneous disorder that tends to occur 24 to 48 hours after resection of a posterior fossa tumor, usually medulloblastoma, ependymoma, or low-grade astrocytoma. Posterior fossa syndrome will develop in up to 25% of patients undergoing surgical resection in the posterior fossa and, in most patients, it will be severe. The pathophysiologic basis for this syndrome is unclear, with many hypotheses proposed. It is likely related to pressure effects on the deep cerebellar nuclei and is more frequently associated with large tumors extending into those areas. Gross total resection (GTR) of most posterior fossa brain tumors is associated with better long-term outcomes, and it is thought that the more aggressive surgical resections for those reasons are associated with the perceived increase in the frequency of this syndrome. The exact clinical patterns of posterior fossa syndrome can vary among patients both in constellation and severity. Usually the disorder includes loss of speech in patients who were capable of talking immediately after surgery. Other symptoms include irritability, which may relate to difficulties in communication, emotional withdrawal, and motor difficulties with ataxia. In a review of 450 children from two large Children’s Cancer Group (CCG) protocols for high-risk and standard-risk medulloblastoma, 107 patients (24%) had posterior fossa syndrome. It was classified as severe in 43%, moderate in 49%, and mild in 8%. As with many neurologic insults, most patients demonstrated significant improvement, although neurologic abnormalities persisted in a large proportion of patients. No uniform diagnostic criteria exist for posterior fossa syndrome, and radiologic imaging with SPECT has failed to define a specific controlling neurologic region.
Venous thrombosis in adults with brain tumors is common, especially when compared with adults undergoing operations for causes not related to brain tumors, and typically requires therapy. A similar predilection to significant symptomatic thrombosis in children with CNS tumors has not been identified. Because of the risks of spontaneous hemorrhage in patients taking anticoagulants, their routine use is discouraged for pediatric patients. When thromboses in this patient population are identified, other precipitating factors such as the presence of a central venous access device are usually evident. CNS hemorrhage is rare in pediatric patients undergoing tumor resection. Use of recombinant factor VIIa has been used successfully when bleeding was difficult to control.
Long-Term Follow-Up
Long-term follow-up is extremely important because neurosurgical sequelae of the treatment of brain tumors exist. The management of hydrocephalus is lifelong, and all survivors of childhood brain tumors who have treated hydrocephalus should undergo frequent evaluations by a neurosurgeon and radiographic evaluation of the patency of the ETV (via specialized MRIs) or of their shunt systems.
A relatively rare but significant complication of radiation therapy and surgery for tumors in the sellar/suprasellar area is moyamoya disease. This condition is manifested by the development of occlusive vascular disease at the base of the skull with subsequent ischemic events and stroke. The management includes surgical revascularization, which is effective in stopping the clinical symptomatology and preventing new ischemic events and is performed in centers with specialized interest in this condition and expertise in the surgical procedure.
Radiotherapy
A detailed section on the fundamentals of radiation oncology is included elsewhere in this text (see Chapter 48 ). The primary goal of this brief discussion is to focus on radiation therapy issues that are specific to pediatric neurooncology. An understanding of the basic principles of radiation therapy is critical because it remains one of the most effective, albeit toxic, therapies for this patient population.
Like chemotherapy, radiation therapy targets dividing cells, one of the hallmarks of cancer therapy. Unlike chemotherapy, however, delivery of radiation therapy is not limited by the BBB. The biologic effects of ionizing radiation are the result of damage to cellular DNA, primarily through irreparable double-strand breaks. Photon radiation can cause damage through direct interaction with DNA or through the formation of free radicals, which damage the DNA. Charged particles can cause damage through direct interactions with the nucleus. This damage results in complex cascades of molecular events, affecting cell cycle checkpoints, apoptosis, DNA damage response, and DNA repair. Tumor cells have lost the regulation required to repair DNA damage before entering cell replication. In contrast normal adjacent cells that receive radiation therapy will repair the damage between fractions. After weeks of continued radiation therapy, normal cells will have repaired themselves, although the repair process is not perfect and accounts for the toxicity to normal brain caused by radiation therapy. Tumor cells, on the other hand, will have accumulated significant damage, leading to cell death. The concept of fractionation is the hallmark of radiation therapy.
Technical Aspects of Radiation Therapy
Radiation therapy technology has advanced continually since the discovery of x-rays. The most commonly used modalities in the treatment of CNS tumors are photon radiotherapy and proton radiotherapy. Both these forms of irradiation provide the same treatment dose to the tumor tissue, and therefore their differences are not related to efficacy. Rather the differences in the therapeutic beams and the physical properties associated with them result in different dose distributions. The major clinical difference between the two techniques relates to potential toxicities to the normal brain.
Photon Therapy.
Photons are packets of high energy that enter tissue, depositing their energy as they pass through both normal tissue and the tumor, eventually exiting the brain. The presence of an exit dose is one of the major differences between this modality and proton therapy. The generation of photon beams is easily achieved with a large array of commercially available machines and, with more than 60 years of clinical experience, photon radiotherapy remains the most widely used form of radiation therapy. Significant advances in this modality have occurred through the development of better imaging (MRI and functional imaging such as PET and metaiodobenzylguanidine), planning algorithms, and machine delivery that has allowed the radiation oncologist to target tumors more accurately and to minimize the dose delivered to normal tissue.
Opposed Lateral Fields.
Used largely before 1990, opposed lateral fields typically rely on two wide beams, usually one from the left and the other from the right. This approach is no longer considered the standard of care in children with a brain tumor because of excessive toxicity to normal brain tissue. At a minimum conformal fields should be used.
Conformal Radiation Therapy.
The conformal radiation technique uses the principle of tumor volume definition through MRI and CT scans. In the treatment of CNS tumors, the anatomic localization of the bony structures of the skull using CT scans are fused to the MRI with specialized fusion software. This technique allows accurate tumor definition and submillimeter accuracy in treatment. Radiation therapists choose beam placement to maximize tumor coverage while avoiding normal brain tissue. With 3D conformal radiation therapy, conformal beams are used to shape the dose delivered to the target, and wedges or compensators can be used to optimize the dose distribution. With 3D conformal radiation therapy, variable field weighting and use of different energies (higher beam energies are more penetrating) are additional tools that enable optimization of the dose distribution. With the development of more rapid and powerful computers, the ability to generate a large number of different beam orientations has allowed for diffusion of the radiation dose over normal tissue, thus reducing long-term damage while ensuring complete coverage of the tumor volume.
Stereotactic Radiotherapy.
Stereotactic radiotherapy is a further improvement on 3D conformal therapy by ensuring improved head immobility so that beam configuration may be further reduced without having to worry about head position and tumor location. In the normal delivery of the radiation beam, some head movement may occur; therefore, the target volume must be expanded in all directions to ensure that at no time is part of the tumor outside the targeted area. To overcome this difficulty, a series of techniques have been developed that use head immobilization to ensure less head movement and consequently smaller target volumes. Options for such immobilization include frames bolted to the skull and then fixed to the radiation device, which is the standard procedure for stereotactic radiation surgery (discussed later). The major limitation of this procedure, however, is that radiation therapy is delivered over approximately 6 weeks, and bolting screws into the skull for this length of time is not practical. A second option is the use of a head mask made for each patient, which fits around the face and skull and can sometimes use the ear canals or palate to ensure exact and reproducible fits. A new development is the use of real-time CT scans that constantly reevaluate the position of the skull, with computer software that corrects for changes in head position to reset the beams accordingly. In children the additional dose of radiation therapy from CT scans is not trivial and needs to be considered in the choice of method.
Intensity-Modulated Radiation Therapy.
Intensity-modulated radiation therapy (IMRT) gives radiation therapists the opportunity to modulate the intensity of a radiation beam so that instead of uniform dosing throughout a volume, areas of decreased intensity may spare critical structures. For example if a critical nerve runs through or adjacent to a tumor, IMRT allows the radiation therapist to spare the middle, like a doughnut, while still treating the entire surrounding tumor. IMRT is a device that fits onto the gantry of a radiation machine and moves small metal slots in and out of the beam path as it moves in arcs to deliver the required fields. Large metal (macroleaf) and small metal (microleaf) pieces are available. The microleaf collimators allow for a more refined shaping of the field. An important limitation of this technique is the development of hot spots in areas within the field. As a general rule this technique can be added to 3D conformal and stereotactic radiation therapy treatment plans.
Stereotactic Radiosurgery.
Stereotactic radiosurgery (SRS) techniques (e.g., gamma knife, cyber knife, or X-knife) use a single fraction (or occasionally several fractions) rather than the prolonged treatment courses that are standard with radiation therapy. As the names imply in these techniques, all develop a focused beam of energy that covers a tight volume and causes the cells within the volume to die. Unlike traditional radiation therapy, in which normal cells recover between successive doses while tumor cells do not, the principle for this technique is similar to focusing sunlight with a magnifying glass to burn a small area. Because of the significant toxicities possible with a technique designed to kill a targeted area, a specific volume that excludes normal adjacent structures is critical. To achieve this requirement, after induction of general anesthesia, the head is often bolted into a metal head frame, and then the metal frame is bolted to the radiation device. In this way no additional or unintended movement can occur that would result in the targeted beam missing part of the tumor and damaging uninvolved adjacent normal brain tissue. New methods that can avoid the need for fixed head localization are being developed.
Proton Radiotherapy.
The use of proton beam radiation therapy as an alternative to high-energy x-rays (photons) has the potential to limit some of the late effects of radiation therapy by reducing the exposure of normal tissue to the radiation. Physically, photon beams deliver their maximum radiation dose near the surface, followed by a continuously reducing dose with increasing depth. Tissues outside the target area receive an exit dose of the radiation beams. For example when a single posterior field is used to treat the spinal axis, critical organs along the path such as the heart, lung, bowel, and ovaries may receive significant exposure. In contrast in proton beam radiotherapy, while the charged particles—namely, protons—move through tissue, they ionize particles and deposit their radiation dose along the path. The maximal dose, called the Bragg peak, occurs shortly before the point of greatest tissue penetration, which is dependent on the energy of the proton beam. Because the energy can be precisely controlled, the Bragg peak can be placed within the tumor targeted to receive the radiation dose. Because the protons are absorbed at this point, normal tissues beyond the target receive little irradiation. Proton beam radiotherapy is becoming more readily available but remains a more limited and costly modality. Children most likely to benefit from proton beam treatment are those with favorable or curable brain tumors such as craniopharyngiomas, medulloblastomas, LGGs, ependymomas, and GCTs. With the increasing complexity of radiation planning, a dedicated pediatric radiation oncology team is required to ensure appropriate care for the pediatric patient.
Toxicity of Radiation Therapy
Even with the significant advances in the highly precise delivery of radiation therapy, a number of circumstances limit its usefulness for children. Humans achieve their maximum number of brain cells shortly after birth. From that time forward, a steady loss of cells continues throughout life. While we age our neurocognitive development is related to the development of new connections and interactions between cells, not the addition of cells. For reasons that are poorly understood, irradiation can affect not only the proliferation of new cells in infancy but also the ability of cells already present to form or maintain connections in children and adolescents.
Late effects of radiotherapy in the treatment of children with brain tumors include neurocognitive sequelae, ototoxicity, hormonal dysfunction, vascular complications, growth disturbance, and secondary malignancies. The severity of these effects depend upon many factors. The three major factors that determine the severity of the impairment after radiation therapy are the age of the patient, the volume of the brain to be irradiated, and the dose of radiation therapy required.
- 1.
Age . Because the age of the patient at diagnosis is not mutable, simply withholding radiation therapy until a child has had an opportunity to grow older would reduce the long-term morbidity, but this approach may allow a tumor to recur. As such, the decision between accepting toxicity or foregoing efficacy is not uncommon in pediatric practice.
- 2.
Volume . Although neurocognitive development is most active from birth until the age of 3 years, significant development occurs up to the age of 10 years and even into adulthood, which implies that radiation therapy to large parts of the brain can cause detrimental effects on cognition throughout life. Because the volume of brain to be irradiated is determined by the extent of tumor spread, radiation therapists must treat the required volumes with all the associated long-term morbidity or reduce the volume to be treated with a corresponding reduction in the efficacy that radiation provides.
- 3.
Dose . The third factor related to radiation toxicity is the dose used. Because most brain tumors require the use of maximal tolerated doses to have a significant clinical impact on outcome, reducing the dose would again require a tradeoff between toxicity reduction and efficacy reduction.
Further discussion of the toxicities of radiation therapy is found in Chapter 47 .
Chemotherapy
The approach and use of chemotherapy, like radiation therapy, does not differ significantly for most children with brain tumors when compared with children with other cancers. Similarly the effects of antiseizure medications on chemotherapy mirror those of other patient populations, and agents susceptible to altered metabolism by enzyme-inducing anticonvulsants, such as irinotecan, require appropriate dose adjustments or a switch to a nonenzyme-inducing anticonvulsant. This requirement has resulted in the development of many pediatric treatment regimens modified from adult studies. The increasing recognition of the unique nature of pediatric tumors in general and pediatric brain tumors specifically has led to the development of pediatric-specific preclinical models of cancer. Incorporation of information from these models is likely to be slow as different combinations of chemotherapy, biologic therapies, and radiation therapy undergo evaluation. The need for drugs to cross into the brain raises questions about agents that showed no activity in prior clinical trials. Although the routine approach was to discard such agents as inactive, the ability to modify such agents to improve their CNS penetration has resulted in the need to retest some of these chemotherapeutic classes.
Blood-Brain Barrier
The BBB results from the tight junctions of endothelial cells and astrocytic projections surrounding the brain that limit the penetration of substances, especially infections and inflammatory responses, from gaining access to the CNS. A slightly different barrier exists between the blood and CSF (called the blood-CSF barrier), although the primary role of the two systems remains the same—to isolate the brain from the entry of as many foreign chemicals and pathogens as possible.
While tumors develop, they grow and invade normal structures, which can disrupt the BBB. Tumors also need to secure a blood supply, which can be achieved through the secretion of a large number of cytokines, of which the best characterized is VEGF. Before its discovery in stimulating angiogenesis, this molecule was initially discovered as vascular permeability factor (VPF) because of its ability to open up endothelial junctions, allowing for changes in fluid shifts. The secretion of VEGF (VPF) by tumors is responsible for significant peritumoral edema and leakage of the BBB. For reasons that remain poorly understood at present, many tumors, or areas within tumors, do not demonstrate disruption of the BBB, making their detection on contrast-enhanced MRI scans more difficult.
General principles of chemotherapy administration in tumors of the CNS are similar to those of other tumors of the body. The CNS lacks a lymphatic system and, because of the presence of the BBB, extraneural metastases are uncommon. Thus the goal of treatment remains focused on the brain and spine. Some agents may not fully penetrate through the BBB, depending on the characteristics of the drug and local breakdown of the BBB. Although the chemical structure of compounds should be an important consideration in their predicted ability to penetrate the BBB, and thus have the potential for clinical activity, many hydrophilic drugs have demonstrated activity in brain tumors. However some hydrophobic agents, which should easily traverse the BBB, do not traverse it. Even with extensive knowledge of the hydrophobicity of a drug, one cannot predict with certainty whether it will have activity in CNS tumors. The platinum drugs, for example, which would not be predicted to penetrate into the brain significantly, are active agents for various tumors in the brain and spine. This outcome may in part relate to the breakdown of the BBB around tumors, resulting in penetration of drugs into restricted areas. One important variable that can significantly affect BBB penetration is the degree of protein binding. To overcome this problem of drug delivery, direct application of drugs into surgical cavities or cysts is possible. Hydrostatic pressure gradients moving from tissue into an empty cavity draw most of the drugs away from the tumor and likely account for the limited activity of chemotherapeutic agent–impregnated wafers along the resection margin.
Intrathecal or Intra-Ommaya Chemotherapy
Intrathecal administration of chemotherapy can overcome the blood-CSF barrier and is of importance in tumors with a predilection to seeding of the brain and spine. This technique is not safe in patients with obstructed CSF flow, but for patients without this problem who also lack diversional shunts that would draw the drugs out of the CSF spaces, high concentrations can be delivered. Because repeated access to the lumbar spine can be uncomfortable, insertion of an Ommaya or Rickham reservoir may reduce the difficulties of repeated administration. To assist in the delivery of chemotherapeutic agents into the CSF, insertion of reservoirs that sit on top of the skull or in the subcutaneous tissue of the abdomen or flank can make repeated administration more practical. Although a number of new agents have been investigated for intrathecal or intra-Ommaya/Rickham administration, including busulfan, etoposide, and mafosfamide, overall a limited number of agents may be safely administered to this compartment, including standard intrathecal agents used in leukemia (e.g., methotrexate and cytarabine), etoposide, topotecan, and liposomal cytarabine.
An important approach to increasing the penetration of chemotherapy into the brain has been the use of high-dose systemic therapy followed by stem cell rescue. The efficacy of this approach and management of the associated toxicities continue to be an area of significant study (discussed later).
Newer methods of targeting penetration of drugs into the CNS include BBB disruption agents. These agents are in clinical trial and are designed to temporarily open up the tight junctions protecting the CNS. They are typically administered just before the active anticancer agent is administered. Although this approach is promising and deserving of additional evaluation, a common problem to date has been the opening of the BBB in normal areas of the brain, resulting in greater toxicities to uninvolved areas. In a similar approach new lipophilic carrier molecules have been designed to help transport drugs across the BBB, and further advances in these areas are expected.
Convection-Enhanced Delivery
With the movement of fluids away from areas of high interstitial pressure to areas of low interstitial pressure, passive diffusion of drugs deep into tumors is unlikely to occur in sufficient concentrations to be effective. To overcome this problem developments in convection-enhanced delivery have been reported. These techniques require the implantation of small catheters that can be tunneled under the scalp, which then penetrate the solid tumor parenchyma or adjacent brain. By injecting drugs under high pressure, these agents move through the interstitial space between cells, giving the drugs an opportunity to kill tumor cells, even in critical areas of the CNS such as the pons. Because tumors usually penetrate along the pathway of least resistance, similar to fluids under pressure, this technique allows the drug to spread out in a fashion similar to that of the infiltrating tumor. A number of candidate molecules designed for convection-enhanced delivery are being developed. This technique should be equally well suited to small-molecule inhibitors, chemotherapeutic agents, biologic drugs (including large protein molecules), and gene vectors. Advances in drug packaging may permit control of drug delivery, improving the activity of this approach.
Novel Chemotherapeutic and Biologic Agents
The revolution in the molecular classification of adult and pediatric tumors has significantly advanced our understanding of pathways implicated in tumor initiation, progression, and metastases. These pathways have also become important targets for new therapy approaches that are included in the realm of chemotherapy but differ in many fundamental ways. The unique mechanism of action of these inhibitors, and their lack of typical chemotherapy-related toxicity (e.g., myelosuppression) make them ideally suited for combination with traditional chemotherapy and radiation therapy. In addition to classic cytotoxic agents, the use of agents that modulate the epigenome, such as histone deacetylases, which modify DNA methylation, a process critical in gene regulation, may allow apoptotic or differentiation genes to be reactivated while turning off proliferative pathways.
The development of new formulations of old drugs also deserves comment. Many drugs that were tested in children with tumors of the CNS did not demonstrate activity, which may have been the result of poor penetration or unknown pharmacokinetics. Modifications to older agents such as doxorubicin by pegylation and liposomal encapsulation will require additional clinical testing. Even vincristine, which is commonly used in pediatric patients with CNS tumors despite a lack of clear data demonstrating its activity in these diseases, is being redeveloped to improve its potential activity.
Numerous targeted agents are now available, and each will require some early pediatric clinical experience regarding dosing and tolerability. Unfortunately most biologic agents are unlikely to possess significant single-agent activity or resistance will develop, although some exciting responses have been observed in a number of different pathways, including SHH and BRAF. Many of these drugs may be better at slowing tumor progression and will need to be used in combination with other molecular inhibitors, radiation therapy, and chemotherapy. These targets may also have important roles as prognostic markers, as well as therapeutic targets.
Small-Molecule Inhibitors.
The sequencing of the human genome and the identification of a number of critical signaling pathways, especially the receptor tyrosine kinases, have provided the basis for a whole new class of anticancer agents. Normal cells transmit signals from the external environment to the nucleus via receptor tyrosine kinases. These molecules sit on the cell surface and homodimerize or heterodimerize in the presence of ligand, which results in a conformational change in the intracellular domain of the receptor. This process allows a phosphorylation event on the cytoplasmic component of the receptor that begins a complex cascade that results in the alteration of cell function. Many tumors use these receptors or their pathways to drive cell proliferation and migration, as well as decouple cell repair and apoptosis.
The activation of receptor tyrosine kinases results from the phosphorylation of a tyrosine residue in the intracellular domain of the receptor. This process can be blocked by the steric interference of small molecules designed to fit into the phosphorylation pocket. By carefully designing the shape of these molecules, the ability to define the specificity of these drugs to related receptors means that some inhibitors can disrupt only a single receptor or entire families of receptors. A number of experimental studies of these inhibitors have been tested in children with brain tumors, including those targeting the platelet-derived growth factor receptor (PDGFR) and ras pathways. One problem with small-molecule inhibitors, as with other agents targeting the CNS, is the need to get drugs across the BBB; small molecules are ideally suited for this purpose, but efflux pumps are present that may expel the agents, resulting in limited activity. Unfortunately this mechanism must be evaluated on a drug-by-drug basis.
Immunotherapy
Although the brain and spine are considered immunologically privileged sites, the presence of lymphocytic infiltrates in many brain tumors suggests that activation of the immune system is possible. Significant attempts to activate the immune system against these tumors, especially malignant gliomas, have followed two main approaches. Some groups have attempted to increase the immune activation of lymphocytes by cytokine stimulation of the innate immune system. In the second approach a patient’s own tumor is required to generate tumor-specific immune-activated cells. While more is learned about how immune cells interact with and are activated by tumors, opportunities to develop immunotherapies may become more feasible, and some early encouraging results are already being reported.
Gene Therapy
The use of gene vectors for the treatment of brain tumor allows for the expression of a large array of different molecules. Many experimental approaches have focused on modulators of the immune response. An important component in the expansion of these approaches will be the identification of different vectors, as well as improvement in direct delivery of large molecules directly into the brain to bypass the BBB.
Antiangiogenic Agents
During the past few decades the role of angiogenesis in the development of cancer has evolved from a novel hypothesis to a fundamental area of research and therapeutic intervention. Although not brain tumor–specific, angiogenesis has been a hallmark of the progression of malignant gliomas. A large number of antiangiogenic inhibitors are now in clinical trials and have focused on targeting the cytokine vascular endothelial growth factor, ; other inhibitors of the angiogenic cascade are being tested as well. Although bevacizumab has been extensively tested in adults with malignant gliomas, its role in pediatric tumors remains unclear. A developing area ideally suited for pediatric patients has been the use of oral antiangiogenic chemotherapy. The principle behind this approach is the use of very-low-dose chemotherapy that targets dividing endothelial cells rather than tumor cells. A number of low-dose chemotherapy approaches are being tested, and preliminary results are encouraging.
Suppression of Tumor Resistance
The innate resistance of many tumors to cytotoxic agents, especially those of glial origin, is well documented by the near-complete and rapid progression of HGGs after upfront radiation and chemotherapy. Tumors can use a number of pathways to avoid cell death when confronted with DNA-damaging agents. Temozolomide has become widely used in conjunction with radiation therapy in adults and has demonstrated clear activity in these patients. Although this combination has demonstrated prolongation in time to progression of the disease, in the vast majority of patients the disease will eventually progress as resistance mechanisms are activated. To help overcome this problem recent clinical trials have begun testing molecules that can bind and inactivate the enzymes responsible for resistance. One such example is O(6)-benzylguanine (O(6)-BG), a small molecular compound that can bind the enzyme O(6)-methylguanine-DNA methyltransferase (MGMT), which functions to remove the methyl group on the O(6) position of guanine after temozolomide treatment. By treating the patient in advance with O(6)-BG, all free MGMT can be consumed, at which point administration of temozolomide can damage the DNA. Although still early in pediatric testing, this approach offers considerable opportunity. Additional resistance pathways have been identified in pediatric and adult brain tumors that may guide therapeutic approaches, as well as the development of new inhibitors of the resistance pathways. For example mutations within the PMS2 mismatch repair gene, which can lead to tumorigenesis and treatment resistance, may be influenced by the addition of retinoic acid.
High-Dose Chemotherapy with Stem Cell Rescue
The principles of high-dose chemotherapy and stem cell rescue are similar to those for other malignant diseases. Many brain tumors, especially those of primitive neuroectodermal origin, such as medulloblastoma and CNS PNETs, have demonstrated dose-dependent chemotherapy responses. This technique has therefore been used extensively in children with relapsed disease after standard upfront therapy and in young children and infants. In light of the long-term neurocognitive effects of radiation therapy for young children, particularly for diseases that require craniospinal radiation therapy, high-dose chemotherapy with autologous stem cell rescue (ASCR) currently serves as the backbone treatment in many infant protocols around the world. Although the conditioning regimens, disease histologies, and patient characteristics have differed across multiple clinical trials and retrospective studies, this approach has demonstrated positive results in patients who could achieve minimal residual disease prior to transplant. The addition of this modality with other therapies remains to be tested and may influence the utility of this approach in persons without minimal residual disease or the ability to maintain the disease-free state. The role of high-dose chemotherapy with stem cell rescue is debatable, however, in the recurrent setting. The evaluation of patients after transplantation can be complicated by the presence of therapy-related signal changes on MRI scan, including heterogeneously enhancing lesions, often causing clinical symptoms related to their location. Although these lesions can appear consistent with disease progression early after transplantation, they do not progress and need to have long-term follow-up with regard to their clinical significance.
Pediatric Brain Tumors
Gliomas
Glial tumors are usually classified according to the type of glial cell that constitute the tumors—astrocytomas, ependymomas, and oligodendrogliomas. Each is further divided by morphologic features, degree of invasiveness, and location and is assigned a grade ranging from I to IV as the features of malignancy increase. In pediatrics, the grading of astrocytomas has been defined by the WHO or St. Anne-Mayo system and is predictive of patient survival. In pediatrics, the modified WHO classification of CNS tumors has become the standard classification system (see Table 57-3 ). Astrocytomas can be classified as low grade (WHO grades I and II) or high grade (WHO grades III and IV). LGGs may consist of relatively pure tumors such as a juvenile pilocytic (grade I) or fibrillary (grade II) astrocytoma or mixed populations of both glial and neuronal lineages, such as ganglioglioma or glioneurocytoma. The classification of several other subtypes is still being debated. Although the differentiation of HGG from LGG is universally used and based on degree of atypia, mitoses, necrosis, and vascular proliferation, with greater molecular definition of astrocytic tumors, the need to separate grade I from II and grade III from IV tumors regarding treatment and prognosis is increasing. Pediatric gliomas can also be discussed in the context of location, rather than grade. This approach recognizes some of the unique aspects of the environment in which tumors of similar histologies can grow and its effect on treatment and prognosis ( Box 57-2 ).
Astrocytic tumors
Pilocytic astrocytoma
Pilomyxoid astrocytoma
Diffuse astrocytomas (fibrillary, protoplasmic, gemistocytic)
Pleomorphic xanthoastrocytoma
Subependymal giant cell astrocytoma
Oligodendroglial and mixed glial tumors
Oligodendrocytoma
Oligodendroglioma
Oligoastrocytoma
Mixed glial-neuronal tumors
Gangliocytoma
Ganglioglioma
Dysembryoplastic infantile astrocytoma and dysembryoplastic infantile ganglioglioma
Dysembryoplastic neuroepithelial tumor
Special locations that are often not biopsied
Optic pathway gliomas
Tectal gliomas
Cervicomedullary gliomas
Low-Grade Glioma
Supratentorial, Cerebellar Pilocytic, and Other Low-Grade Astrocytomas.
LGGs represent the most frequent group of brain tumors that develop during childhood. According to the latest statistical report from the Central Brain Tumor Registry of the United States, the annual incidence of pediatric LGGs in the United States is 2.1 per 100,000 persons. PAs, which make up the majority of LGGs, are well-circumscribed tumors classified as WHO grade I. These tumors were formally referred to as juvenile PAs but are now classified simply as PAs. Grade I tumors are the most common LGGs found in children, representing 20% to 30% of all childhood brain tumors. PAs typically appear in the first two decades, with no clear gender predominance. They usually grow slowly, although their presentation can occur as acute deterioration as a result of obstructive hydrocephalus. NF-1 is the best example of a condition associated with an increased risk of PA in up to 15% of these patients. The localization of PAs in the context of NF-1 and their improved long-term prognosis is well established, although the molecular basis for this difference is unclear. Other predisposing factors such as common cytogenetic abnormalities are uncommon. Low-grade astrocytomas typically lack epidermal growth factor receptor (EGFR) amplification, although defects in the BRAF pathway have recently been reported and are present in the majority of cases.
Clinical Presentation.
Low-grade astrocytomas in children have a varied course, ranging from dissemination and persistent recurrence to spontaneous regression without therapy, and they behave differently from low-grade astrocytomas in adults. The biologic factors that account for these differences remain investigational, although telomere length may play an important role. PAs commonly occur throughout the brain, including the optic pathways, optic chiasm–hypothalamus, thalamus and basal ganglia, cerebral hemispheres, cerebellum, and brainstem (dorsally exophytic brainstem glioma). PAs of the spinal cord are less common.
The spectrum of clinical manifestations of a PA depends on the site of origin, its size, the age of the patient, and the presence of raised ICP. Cerebellar PAs and dorsally exophytic brainstem PAs usually present with symptoms of increased ICP, such as headache, nausea, and vomiting. Children may also present with a relatively long history of progressive focal neurologic deficits, including gait disturbance, and infants may present with progressive secondary macrocephaly. Signs of chronicity such as bone remodeling, scoliosis, or hemihypertrophy may be present, depending on the primary tumor location. The diencephalic syndrome is unique to low-grade astrocytomas, both PA and pilomyxoid astrocytoma, is typically seen in infants whose tumors arise from the hypothalamus or optic pathways, and consists of emaciation, emesis, euphoria, and normal linear growth. Although many other deep-seated low-grade glial tumors cannot be resected, patients with diencephalic syndrome appear to have a worse prognosis, suggesting that subtle biologic differences among these tumors and other PAs may exist. Leptomeningeal dissemination is associated at diagnosis in 3% to 5% of cases.
Imaging and Histology.
The typical MRI appearance of a grade I astrocytoma is that of an intensely homogeneous, well-circumscribed, contrast-enhancing lesion with minimal surrounding edema. Lesions are typically bright on both T1- and T2-weighted images. Tumoral cysts are more prevalent in the cerebellum than in the cerebrum ( Fig. 57-18 ) and often possess a contrast-enhancing mural nodule. Apparent diffusion coefficient imaging may be useful in differentiating PAs from higher grade astrocytomas. Imaging characteristics can help with the differential diagnosis of low-grade lesions preoperatively but are not specific enough to be used without biopsy confirmation.
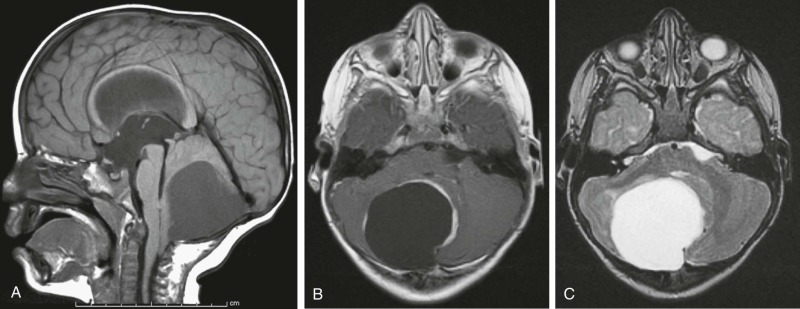
Histologic examination reveals a biphasic pattern, with a compacted component containing bipolar cells ( Fig. 57-19 ) and Rosenthal fibers and a loose cellular array containing microcysts and eosinophilic granular bodies. Rosenthal fibers and eosinophilic granular bodies are pathologic hallmarks of pilocytic astrocytomas, although they can be observed in other diseases of the CNS. Rosenthal fibers are brightly eosinophilic, hyaline masses composed of alpha-B-crystalline and are best seen on tumor smear preparations ( Fig. 57-20 ). Eosinophilic granular bodies are globular aggregates within astrocytic processes and are also best visualized with smear preparations. PAs stain intensely with the GFAP immunoreagent. Invasion of the overlying meninges and adjacent brain parenchyma is commonly observed. Mitoses are rare, and the MIB1 labeling index is usually lower than 4%. Although the pathologic criteria for anaplastic astrocytoma include the identification of mitoses, their presence in PAs does not indicate a higher grade. Inexperienced pathologists can often misinterpret these mitoses, resulting in a diagnosis of a malignant rather than an LGG. Similarly PAs can have vascular proliferation, a hallmark of glioblastoma multiforme (grade IV astrocytoma), although, again similar to that of the presence of mitoses, this does not indicate transformation to a more malignant phenotype. Rarely PAs can present with diffuse leptomeningeal dissemination, especially in the variant of pilomyxoid astrocytoma. Characteristic of the unique biology of PAs, each of the metastatic lesions continues to behave as a low-grade astrocytoma with a slow, indolent course. These tumors therefore are not difficult to treat as a result of their metastatic phenotype but rather as a result of their slow, persistent recurrences.
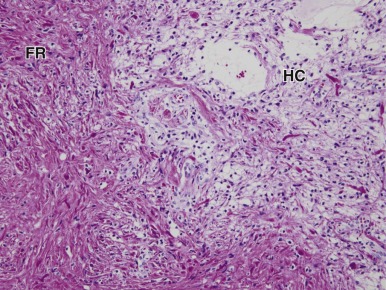
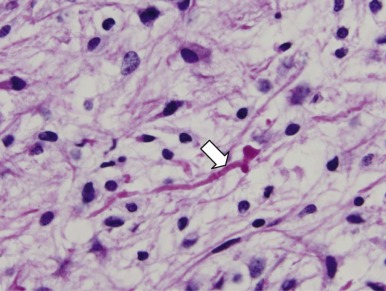
Molecular and Genetic Characteristics of Pediatric LGGs.
Recent efforts in the characterization of genomic alterations in pediatric LGGs have significantly expanded our understanding of the biology of those tumors. Genomic alterations of the BRAF gene, resulting in alteration of the MAPK pathway, are prominent in pediatric but not adult LGGs. Other genomic alterations affecting PI3K/AKT, EGFR, PDGFRa, FGFR1, TrkB, MybL1 and VEGF signaling pathways have been described in a subset of pediatric LGGs.
Importantly pediatric LGGs present distinct molecular alterations compared with adult LGGs. Tumor protein p53 (TP53) mutations are frequent in adult LGGs (up to 60% to 70%) but are rare in pediatric LGGs. Deletion of 1p and 19q is the most frequent copy number alteration in adult oligodendrogliomas. In contrast very few 1p-19q codeletions have been reported in pediatric LGGs. IDH1 and IDH2 mutations occur in about 70% of adult LGG, whereas these mutations are very rarely described in the pediatric population.
BRAF Truncation-Duplication.
The high incidence of LGGs in patients with NF-1 prompted initial investigation into the role of the MAPK pathway in pediatric LGG tumorigenesis. Early comparative genomic hybridization studies performed on pediatric LGGs, especially PAs, identified a significant recurrent gain of the 7q34 region containing the BRAF locus. This region is amplified in 50% to 90% of pediatric PAs, with the highest frequency in tumors of the posterior fossa and of the hypothalamic/chiasmatic region and less frequently in fibrillary astrocytomas and oligodendroglial tumors, and they are thought to have a better prognosis than other LGGs. Further genomic studies found that the 7q34 gain corresponded to a BRAF duplication and KIAA1549 insertion. In vitro validation showed that this gene product is constitutively active, leading to downstream upregulation of effectors of the MAPK pathway, MEK and ERK. One short form of the KIAA1549-BRAF fusion induces anchorage independent growth in vitro. Also short-term cultured pediatric LGG lines showed significant diminution of cell proliferation rate using pharmacologic inhibitors of MEK1 and MEK2. Other partners of BRAF fusion have recently been identified that involve either SRGAP3 or FAM131B, all causing MAPK pathway activation. Although break points between BRAF genes differ in those variants, they all result in the loss of the N-terminal inhibitory domain of BRAF , leading to the constitutive activation of the BRAF kinase. Recent functional studies using BRAF fusion variants have highlighted the importance of the context in which this genomic alteration drives tumor growth. Kaul et al and colleagues demonstrated that in vivo transfection of the BRAF fusion transcript in mature astrocytes was not able to induce glioma tumors, whereas transfected neural stem cells were able to develop tumors. Another recent study comparing the overexpression of the BRAF-KIAA transcript in neural stem cells of different regions within the brain showed that tumors appeared only in neural stem cells located in the third ventricular region.
BRAF V600E Point Mutation.
BRAF V600E mutation has been described in a variety of cancer subtypes including melanoma, colorectal cancers, papillary thyroid carcinoma, non–small-cell lung carcinoma, and leukemia. This mutation enhances BRAF kinase activity and leads to the constitutive activation of the MAPK pathway. In contrast to BRAF truncation-duplication, which is strongly associated with grade I histology, the BRAF V600E mutation occurs more frequently in grade II pediatric LGGs (PLGGs), especially fibrillary astrocytomas, gangliogliomas, pilomyxoid astrocytomas, and pleomorphic xanthoastrocytomas (PXAs). V600E mutation was also described as transforming fibroblasts in vitro, suggesting that this specific genomic alteration drives cell proliferation in a subset of PLGGs. Interestingly a recent study showed that BRAF V600E mutation promotes neural stem cell transformation followed by senescence, which may parallel the natural history of PLGGs.
Other Genomic Alterations Affecting Key Pathways.
In addition to the MAPK pathway, other pathways such as PI3K/AKT/mTOR, EGFR, SHH, and VEGF have been described to be altered in PLGGs. Although PTEN deletions and p16 deletions were previously identified in anaplastic astrocytomas and therefore associated with a more aggressive phenotype of astrocytic tumors, a recent study of 32 PLGGs showed that 44% had PI3K/Akt/PTEN/mTOR activation, mostly through PTEN promoter methylation. Additionally BRAF fusion transcript transfection in vivo is associated with mTOR pathway activation through the S6-kinase cascade. EGFR amplification, assessed by genomic hybridization and fluorescence in situ hybridization, was described in a subset of disseminated PLGGs, suggesting that the EGFR pathway may play a role in a fraction of invasive LGGs. Evidence of WNT pathway activation in a subset of PAs, especially in young children, has been highlighted by a recent study showing that the patched (PTCH1) gene, coding for the PTCH receptor, was highly expressed in a subset of PAs, especially in patients younger than 10 years. PAs might also carry abnormal function of the VEGF pathway, supported by the observation that vessel architecture of those tumors is often immature and unstable, comparable with that of HGGs. The active phosphorylated forms of the VEGF receptors 1, 2, and 3 have been shown to be highly expressed in tumor vasculature in PAs.
Recently genomic alterations of the transcriptional activator MYB, a known oncogene in other tumor subtypes, especially T-cell acute lymphoblastic leukemia, have been identified in PLGGs. MYB amplification and focal deletions have been described in fibrillary astrocytomas and in angiocentric gliomas, respectively.
Management.
Surgery is the mainstay of therapy for most pilocytic and other low-grade astrocytomas. GTR is often curative, even though residual microscopic disease may often be left behind. Radiation therapy and chemotherapy are typically not required as part of upfront therapy after a complete resection. Surgery is also an effective method of seizure control in patients with LGGs, especially those of the temporal lobes. PLGGs in more eloquent areas may not be amenable to surgical resection. Because the OS even of nonresectable PLGG is very high, surgical resection should not be attempted when a significant risk of morbidity exists. Unfortunately even in patients with complete resection, the presence of a tumor and surgical intervention can be associated with some long-term adverse effects. PAs of the optic pathway in patients with NF-1 do not require surgical confirmation unless atypical radiographic or clinical features are present. Patients with tectal gliomas require CSF diversion but do not benefit from biopsy; they can be diagnosed on the basis of the presence of hydrocephalus and MRI appearance of the lesion alone. In the largest prospective series of patients with PLGG stratified to observation, chemotherapy, or radiation therapy, the overall outcome of these patients was similar to that of patients reported in other independent series and is an important milestone in the assessment of more than 1000 patients with PLGG.
Progressive or unresectable PAs, or those arising in infants or children that cause alterations of vision or other neurologically relevant symptoms, may require adjuvant treatment. Chemotherapy is assuming an increasingly important role in the management of unresectable and/or progressive LGGs, diencephalic LGGs in younger patients, and other unresectable tumors ( Table 57-5 ). Various combination regimens, such as carboplatin and vincristine or thioguanine, procarbazine, lomustine (CCNU), and vincristine (TPCV) have produced consistent, durable responses, reviewed by Perilongo. Monthly carboplatin is more easily administered but may have less activity and requires additional study.
| Treatment | No. of Patients | Objective Response, % (CR + PR) | Overall Response, % (CR + PR + SD) | EFS or OS |
|---|---|---|---|---|
| Carboplatin | 80 | 2 CR, 17 PR | 2 CR, 17 PR, 4 MR, 46 SD | 72% 3-yr EFS in patients with NF-1; 62% 3-yr EFS in patients without NF-1 |
| Carboplatin | 12 ND | 4 PR | 4 PR, 6 SD | |
| Carboplatin | 4 ND, 2 PD | 6 SD | ||
| Carboplatin | 13 ND/PD | 1 CR/PR | ||
| Iproplatin | 15 ND/PD | 1 CR/PR | 1 CR/PR, 9 SD | |
| Cyclophosphamide | 15 ND | 1 CR | 1 CR, 9 SD | |
| Cyclophosphamide | 1 PD, 3 ND with leptomeningeal dissemination | 2 PR/MR, 2 SD | ||
| Ifosfamide | 6 | 1 PR | 1 PR, 3 SD | |
| Temozolomide | 21 PD | 1 PR | 1 PR, 20 SD | |
| Temozolomide | 13 PD | 2 CR, 3 PR | 2 CR, 3 PR, 3 MR, 4 SD | 57% 3-yr EFS |
| Temozolomide | 10 ND, 20 PD | 3 PR | 3 PR, 1 MR, 25 SD | 51% 2-yr PFS and 17% 4-yr PFS |
| Temozolomide | 2 PD | 2 SD | ||
| Methotrexate | 10 PD | 2 PR | 2 PR, 5 SD | |
| Topotecan | 2 PD | 1 PR | 1 PR, 1 SD | |
| Topotecan | 11 PD | 5 SD | ||
| Etoposide | 14 PD | 1 CR, 4 PR | 1 CR, 4 PR, 3 SD | |
| Etoposide | 12 ND | 6 PR/SD | ||
| Vincristine–actinomycin-D | 24 ND | 3 PR | 3 PR, 6 MR, 15 SD | |
| Vincristine-carboplatin | 123 ND | 105/123 CR, PR or SD | 61% 5-yr PFS | |
| Vincristine-carboplatin | 78 ND | 4 CR, 22 PR | 4 CR, 22 PR, 18 MR, 29 SD | 68% 3-yr PFS |
| Vincristine-carboplatin | 24 PD | 7 PR | 7 PR, 5 MR, 5 SD | |
| Carboplatin-etoposide | 13 ND | 1 CR | 1 CR, 3 MR, 6 SD | 69% patients alive at mean of 30 mo |
| Cisplatin-etoposide | 31 ND, 3 PD | 1 CR, 11 PR | 1 CR, 11 PR, 12 MR, 11 SD | 3-year PFS 100% in patients older than 5 yr, 66% in patients younger than 5 yr |
| Vincristine-etoposide | 11 ND, 9 PD | 1 PR | 1 PR, 4 MR, 9 SD | |
| Tamoxifen-carboplatin | 12 ND, 1 PD | 2/13 PR | 2/13 PR, 9/13 SD | 47% 3-yr PFS, 69% 3-year OS |
| 6-Thioguanine, vincristine, CCNU, dibromodulcitol, procarbazine (TPDCV) | 15 ND (4 patients were treated with other therapy and are not included), 42 ND | 11 PR, 15 CR/PR | 11/15 PR, 15 CR/PR + 25 SD | Median TTP not reached at 79 wk, median TTP at 132 wk |
| Procarbazine, carboplatin, vincristine, etoposide, cisplatin, cyclophosphamide | 85 ND | 36/85 | 74/88; 36 CR or PR, 15 MR, 23 SD | 34% 5-yr PFS, 89% 5-yr OS |
| Carboplatin, etoposide, cyclophosphamide, vincristine, CCNU, procarbazine | 7 ND, 3 PD | 2/10 | 70% CR + PR + MR; 100%, including SD | 70% PFS at 5.6 yr |
| 5-Fluorouracil, vincristine, cyclophosphamide, etoposide | 13 (12 ND, 1 PD) | 6/13, 1 CR, 5 PR | 8/13, 1 CR, 5 PR, 2 SD | 6-yr PFS 67% |
| Vinblastine | 9 (ND with carboplatin allergy; nonprogressive at time of therapy) | 2/9 | 9/9; 1 CR, 1 PR, 5 MR, 2 SD | Median follow-up of patients, 10 mo |
| Thioguanine, procarbazine, CCNU, vincristine | 9 (5 ND with carbo allergy; nonprogressive at time of therapy, 4 PD) | 0/9 | 7/9; 7 SD | 78% progression-free at 13 mo |
| Cisplatin, etoposide, vinblastine | 16 ND | 4/16 4 PR | 9/16 4 PR, 5 SD | 5-yr PFS 56% |
With multiagent combinations, stabilization of tumor occurs in almost 50% of patients, and radiographic response is observed in an additional 40%. Median time to progression is approximately 3 years, and up to 60% of patients will eventually demonstrate tumor growth. The Children’s Oncology Group (COG) recently completed a prospective randomized phase III clinical trial examining outcomes of children younger than 10 years treated with vincristine and carboplatin versus TPCV. The TPCV arm showed a trend toward a superior 5-year event-free survival (EFS) compared with the vincristine and carboplatin arm (52% vs. 39%, respectively), although no statistically significant difference was found. The ability to retreat these patients with multiple regimens has allowed most patients to avoid radiation therapy, especially early in life when the long-term morbidity of this modality is greatest. Although the time to progression may appear short and the overall progression rate of 50% to 60% appears high, these chemotherapy regimens are well tolerated, with few long-term complications. This outcome is in contrast to radiation therapy, which demonstrates a significantly improved response rate (85% response or stable disease) and duration of disease control (longer than 10 years) ; however, radiotherapy also entails significant long-term morbidities, such as neurocognitive, vascular, hormonal, and second tumor risks. Because most children with LGGs will be long-term survivors, this is exactly the population that would benefit from the avoidance of the late effects of radiation therapy. To reduce the volume of normal tissue, stereotactic conformational external radiotherapy, stereotactic radiosurgical techniques, and proton radiotherapy have been evaluated in the treatment of recurrent and progressive PLGG tumors. However more focused delivery of radiation in patients with PLGGs only marginally mitigates the long-term sequelae, which include malignant transformation and second malignancy, vascular injury, and, depending on location, neurocognitive decline, endocrinopathies, and other neurologic deficits. These significant and largely irreversible iatrogenic sequelae, conferred in the treatment of a disease whose natural history is self-limiting in most cases, provide an argument in favor of a radiation-avoidance strategy for children with PLGG.
The overall improved outcome for patients with NF-1 has also been confirmed, indicating that these tumors may have a unique biologic phenotype. Temozolomide (TMZ), an orally active alkylating agent with a favorable adverse effect profile, has been shown to have some activity as monotherapy for adult LGGs. Although it has not been widely studied, it appears to have a low response rate in LGGs in children, with a median time to progression of 6.7 months, although many patients appear to have prolonged stable disease. Responses with TMZ in disseminated low-grade astrocytomas have also been reported, although another alkylator, cyclophosphamide, when given every 3 weeks, lacked significant activity. The role of TMZ in combination with vincristine and carboplatin for PLGGs is currently being investigated. Other investigators have successfully used novel combinations containing 5-fluorouracil. The metronomic application of vinblastine, a mitotic inhibitor, has resulted in some clinical responses or stable disease in children with LGGs who are unable to tolerate carboplatin. Vinblastine is also being evaluated alone and in combination with carboplatin in newly diagnosed patients. Although more information on the activity of these approaches is needed, responses in refractory LGGs have been reported with other metronomic-based chemotherapy approaches, suggesting that angiogenesis may be an important pathway in these tumors. Antiangiogenic approaches, such as bevacizumab, have also been tested with some responses, which is also consistent with the presence of vascular proliferation observed in these tumors. Chemotherapy may also allow for improved surgical resection of previously unresectable lesions and therefore should be continuously reevaluated as a therapeutic option. Tumors treated with chemotherapy, even if they are not smaller, can be easier to resect because of less bleeding and a firmer texture at the time of the procedure. The recent identification of BRAF mutations in the majority of PLGGs offers the potential for targeted approaches to these patients. Phase II studies of the downstream inhibitor of mTOR, called everolimus, have recently been completed in this patient population with encouraging activity. In addition BRAF V600E small-molecule inhibitors are also being evaluated for patients with this specific mutation. Importantly the majority of patients with LGGs have the truncated fusion of BRAF (often referred to as the KIAA1549 fusion because this is the most common translocation). Treatment with a BRAF drug such as sorafenib or other V600E targeted agents would be expected to stimulate tumor growth as a result of a complex feedback loop, not to inhibit tumor growth. Patients with truncated fusions will require therapy that accounts for this biologic pathway, and MEK inhibitors are just entering clinical trials for this patient population.
Although most chemotherapy regimens for LGGs have been reserved for children younger than 10 years, older children seem to derive equal benefit with the use of these regimens, potentially avoiding radiation therapy and the risks of second tumors, vasculopathy, and hormonal dysfunction. The use of dose-intensive chemotherapy has been pilot tested in a small series of children, with results similar to those observed using standard doses.
Radiotherapy is considered to be contraindicated in children with NF-1 and is usually deferred in children with PAs and other LGGs, especially in diencephalic and optic pathway tumors. Even highly focused radiation therapy in these locations cannot avoid the potential cognitive, endocrine, or vascular risks associated with radiation therapy. In spite of this highly focused radiation therapy is effective for LGGs and is without significant marginal failures, suggesting that these lesions have not deeply penetrated into surrounding brain. Long-term concerns about second tumors, hormone dysfunction, and cognitive impact, however, still make this approach questionable for children. Additional late effects of radiation therapy when used in younger children with diencephalic gliomas may include strokes related to a moyamoya-like syndrome.
Prognosis.
The prognosis for surgically resectable tumors is excellent after GTR. For patients with PAs whose tumors can be completely surgically resected, depending on location, the 10-year PFS approaches 90%. Even in patients with incompletely resected lesions, treatment is not always required and, depending on the patient and clinical scenario, observation can be considered unless tumor or symptom progression is documented. The most critical variable in the treatment of PAs is the anatomic location of the tumor. Complete resections are most difficult for tumors located in the brainstem, spinal cord, optic pathways, thalamus, and hypothalamus. As such, the PFS of children with centrally located tumors (e.g., in the optic chiasm, thalamus, or hypothalamus) is less than 50%. Given the favorable toxicity profile of chemotherapy versus radiation therapy in young children, chemotherapy is preferred as the first therapeutic modality in young patients with tumors not amenable to gross total resection. The use of chemotherapy as initial treatment in patients with centrally located or unresectable lesions allows for the delay of radiation therapy until the child is less likely to incur the serious developmental and neuropsychological sequelae of radiation therapy. Patients demonstrating radiographic response to chemotherapy have PFS similar to those whose tumors remained stable. Ultimately the quality of survival depends on multiple factors, including the tumor location, the extent to which the tumor can be resected, timing of any radiotherapy, and adverse effects of surgery, chemotherapy, and radiotherapy. Transformation of PAs into higher grade malignant gliomas is highly unusual.
Optic Pathway Gliomas
Optic pathway gliomas (OPGs) represent approximately 4% to 6% of all primary pediatric brain tumors; the incidence is higher when asymptomatic lesions in patients with NF-1 are also included. OPGs are evenly distributed between boys and girls. These tumors may involve various parts of the optic pathway, such as the optic nerves, chiasm, optic tract, and optic radiations. They may also infiltrate the adjacent hypothalamus and temporal lobes. Optic nerve gliomas are strongly associated with NF-1, although sporadic lesions are not uncommon. OPGs in patients with NF-1 have a more indolent course than those arising in patients without NF-1 and likely represent a different biology than sporadic tumors. Although OPGs may be considered a subset of PAs, their unique features and management necessitate a separate discussion.
Clinical Presentation.
Most OPGs consist of PAs (WHO grade I). The clinical course of OPGs can vary considerably, from an indolent mass in a child with NF-1 to a relatively aggressive, invasive, and expansile diencephalic tumor. Unilateral optic nerve gliomas often present with the classic triad of visual loss, proptosis, and optic atrophy. Optic nerve tortuosity, which can be present alone or in the context of OPGs in children with NF-1, does not necessarily define the presence of disease or the need for therapy. Chiasmatic involvement may lead to unilateral or bilateral visual loss, a bitemporal visual field defect, and obstructive hydrocephalus as the tumor grows dorsally to obstruct CSF flow in the third ventricle. Further invasion into brain parenchyma may result in more pronounced visual field deficits, as well as hemiparesis. Chiasmatic tumors in infants present as large suprasellar masses that may also extend into the hypothalamus and third ventricle, producing hydrocephalus and endocrine abnormalities. In cases of hypothalamic extension, in addition to nystagmus, visual loss, and hydrocephalus, the diencephalic syndrome may occasionally be seen. This syndrome consists of hyperkinesia, euphoria, and emaciation, with preserved normal linear growth. Failure to thrive is a common presentation of this syndrome, but in the context of a number of other conditions that may also cause failure to thrive during infancy, a delay in diagnosis of a brain tumor is not uncommon. Endocrine deficiencies commonly accompany PAs in this location, and these tumors are more likely to have CSF dissemination in association with diencephalic syndrome, in spite of their histopathologic classification as benign. Many patients with NF-1 will have long-standing, subtle ophthalmologic abnormalities, and thus regular visual evaluation is required. For these patients routine surveillance MRI scans are not indicated because many asymptomatic and clinically irrelevant tumors will be diagnosed, creating a treatment dilemma.
Imaging and Histology.
The clinical diagnosis of an OPG is suspected when a child presents with visual impairment, nystagmus, and/or optic atrophy. MRI of the brain or orbits typically shows a solid, cystic, or mixed type of tumor, with strong gadolinium enhancement. The imaging characteristics of LGGs of the optic pathway are similar to those of low-grade astrocytomas in other locations. The typical MRI appearance of a grade I astrocytoma is an intensely homogeneous, well-circumscribed, enhancing lesion with minimal surrounding edema ( Fig. 57-21 ). Lesions are typically bright on both T1- and T2-weighted images. A higher ADC may predict a greater chance of progression. MRI studies and clinical presentation may distinguish an OPG from other childhood tumors that arise in the suprasellar location, such as a GCT or craniopharyngioma. Histologically OPGs are usually grade I PAs or, less frequently, grade II fibrillary astrocytomas. Mixed LGGs have been reported in this region, but the overall therapeutic approach is not altered, because most of these lesions are not resectable at diagnosis. Tumors with an elevated MIB1 labeling index of more than 1% and p53 expression were more likely to be WHO grade II and had a worse outcome compared with tumors with an MIB1 and p53 labeling index less than 1%, all of which were PAs. PAs with a more aggressive behavior had an MIB1 labeling index of 2% to 3% but retained a low p53 labeling index of less than 1%. Like LGGs in other locations, patients with sporadic OPGs have both BRAF V600E and truncated fusion KIAA1549 abnormalities. BRAF abnormalities are rare in patients with NF-1 because of the existence of constitutive activation of the ras/raf/mek/MAPK pathway.
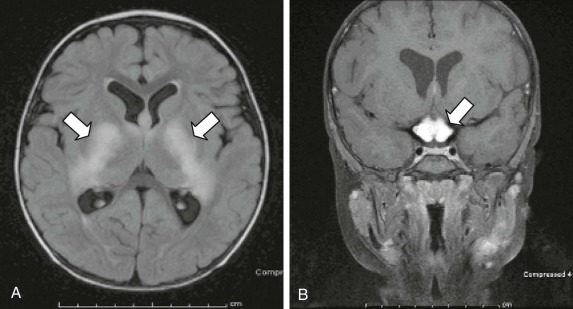
Management.
The unpredictable clinical course of patients with optic pathway tumors has led to controversy regarding the optimal management of these tumors. The clinical course, age of onset, severity of symptoms, size and extent of the tumor, and presence of NF-1 may all affect management decisions. Treatment is frequently started promptly in younger patients, in patients with progressive symptoms, and in those with more extensive CNS involvement, with the paramount concern being preservation of vision. Although progression can be easily quantified on an MRI scan, correlation between MRI findings and visual outcome is poor. The initial treatment of choice is chemotherapy (see Table 57-5 ), which may cause stabilization or regression even in older children and adolescents and is not associated with the neurocognitive decline observed with radiation therapy. Surgery can be used upfront in selected cases, but the tumor may not be removed from the optic nerve without sacrificing vision. Combination therapy with carboplatin and vincristine or with TPCV has been considered to have comparable beneficial effects. TPCV should not be used in children with NF-1 because of the increased risks of secondary tumors associated with alkylator-based treatment. When progression of the tumor occurs after treatment with vincristine and carboplatin, vincristine and actinomycin can be considered for patients with NF-1 to avoid further risks of alkylator therapy. As for other LGGs, a number of new chemotherapy regimens are being developed (see Table 57-5 ) and can include bevacizumab and irintoecan, vinblastine, metronomic therapy, and low-dose cisplatin and etoposide. Current clinical trials targeting the mTOR pathway are under way, as is a phase II trial of lenalidomide. Although delaying radiation therapy is paramount in young children, older patients may also benefit from these chemotherapy approaches and from delaying or avoiding radiation therapy.
A randomized phase III COG study comparing the two regimens, carboplatin and vincristine versus TPCV for the treatment of progressive low-grade astrocytoma in children younger than 10 years, closed to accrual in 2005. The results have shown that both regimens are well tolerated and can delay or obviate the need for radiation therapy in most patients, and that some patients will show improvement in vision with therapy. Newer chemotherapy clinical trials for OPGs combine a number of agents such as TMZ with vincristine and carboplatin, and vinblastine and carboplatin. Although a definitive role for radiotherapy exists for the management of OPG, current trends in treatment favor a delay in the initiation of radiotherapy in young patients. Newer surgical techniques with direct administration of radiotherapy are also being explored and have demonstrated usefulness for selected patients. Unlike LGGs in other areas, optic pathway tumors affect vision directly, and changes in visual fields and acuity can be a better determinant of both disease response or progression than are changes on MRI. Recent consensus criteria for the assessment of visual function in patients with NF-1 have been reported, and these criteria should provide a useful platform for the assessment of therapies that target OPGs.
Prognosis.
Although optic pathway tumors are almost always of low-grade histology, their location often results in serious morbidity. In patients with significant visual deterioration, progressive vision loss can continue for many years after the tumor has become stable on routine surveillance imaging. The growth rate of these tumors, however, often slows during late childhood so that by adulthood, tumors may become quiescent and do not require further therapy. Children with NF-1 have been shown to have a better PFS, although an age of younger than 1 year is clearly associated with a higher risk of tumor progression. Patients with NF-1 appear to be at high risk for the development of moyamoya disease, as well as radiation-induced second malignancies. Patients with NF-1–associated optic nerve gliomas may remain stable for several years. Close observation and symptomatic management are recommended for these patients.
Low-Grade Astrocytomas of the Brainstem
Although most brainstem tumors are DIPGs, approximately 20% of brainstem tumors are low-grade astrocytomas involving the medulla, midbrain, tectum, or cervicomedullary, pontomedullary, or midbrain-pontine junction. Although 20% of nonpontine tumors in these locations can be classified as grade III or IV malignant gliomas, 80% are low-grade glial lesions with a much better prognosis. These lesions differ from diffuse pontine gliomas by their clinical presentation and their imaging characteristics. Early identification of the type of brainstem lesion is critical in the workup, especially for consideration of neurosurgical intervention.
Presentation.
Patients with brainstem lesions can present with various clinical symptoms, depending on the location of the tumor. Medullary tumors are more common than midbrain tumors, and the male to female ratio is approximately 1 : 1. In spite of the eloquent function of the brainstem, most patients with low-grade astrocytomas in this location have an indolent course with subtle neurologic findings. Most commonly patients present with cranial nerve dysfunction or head tilt. Lower motor weakness with subtle hemiparesis is also seen. Most parents have difficulty defining the start of the symptoms and refer to their child as having always been clumsy or weak. Rarely these tumors can be multifocal in nature.
Histology and Imaging.
Most low-grade brainstem tumors are either grade I or II astrocytomas; fewer than 20% are malignant astrocytomas. Imaging characteristics are similar to those of other LGGs; PAs (grade I) tend to be bright on T1- and T2-weighted images and enhance after administration of contrast material. Edema tends to be minimal. Fibrillary astrocytomas (grade II), by comparison, enhance to a lesser degree after administration of contrast material. The considerable overlap and variability in the imaging characteristics of grade I versus grade II astrocytomas, however, prevents accurate diagnosis based on MRI characteristics alone. The histologic features of brainstem low-grade astrocytomas are identical to grades I and II astrocytomas in other locations.
Management.
Brainstem low-grade astrocytomas that remain focal can often be surgically resectable if they possess a plane between the brainstem and tumor. If the tumor is completely resected, these patients are unlikely to need additional therapy. Given the good long-term outcome of this patient population, aggressive surgery should not risk damage in areas with poor tumor boundaries. Incompletely resected brainstem low-grade astrocytomas have a high recurrence rate, and most of these patients will need additional therapy, usually chemotherapy rather than radiation therapy, given the good long-term prognosis of these patients (see Table 57-5 ). White matter tracts are often displaced by these tumors, and preoperative DTI can assist the neurosurgeon regarding the optimal approach to maximize resection while minimizing morbidity in this patient population. Although postsurgical morbidity such as problems with swallowing can be significant, many patients eventually recover function, although this process can take many months and requires extensive physical and occupational therapy. The chemotherapy options for these tumors are identical to those for low-grade astrocytomas in other locations and include vincristine and carboplatin or TPCV chemotherapy. Recurrence and need for retreatment are common, but most tumors will eventually stop growing while these patients enter adulthood.
Prognosis.
Because most patients with a low-grade brainstem glioma will be long-term survivors, it is important that the workup and treatment of these patients be adapted with this likely outcome in mind. Differentiation from DIPGs is usually easily made on the basis of imaging and clinical criteria. Surgical intervention must be based on the expectation that minimal long-term morbidity will result, given the large number of other treatment options available for these patients.
Low-Grade Thalamic Astrocytomas
Thalamic tumors are rare in pediatric patients, accounting for fewer than 5% of intracranial tumors. Most thalamic tumors are unilateral, and approximately 50% are low-grade astrocytomas. Thalamic tumors occur at a slightly older age than do many other LGGs of childhood. Low-grade thalamic tumors present as unilateral or bithalamic in location, which appears to have prognostic significance.
Clinical Presentation.
Thalamic tumors can present with a number of different clinical findings. Raised ICP, tremors, motor deficits, seizures, and mood changes are the most commonly observed presenting symptoms. Unlike in adult tumors, dementia is a rare presenting symptom.
Imaging and Histology.
Imaging characteristics of thalamic tumors are similar to those of gliomas in other locations ( Fig. 57-22 ). Low-grade and high-grade histologies are approximately equally distributed, although pathologic classification of these tumors can be influenced by sampling error from small biopsy specimens. The use of PET or SPECT imaging can help identify areas to be biopsied. Although astrocytic histologies predominate, oligodendroglial tumors have also been identified.
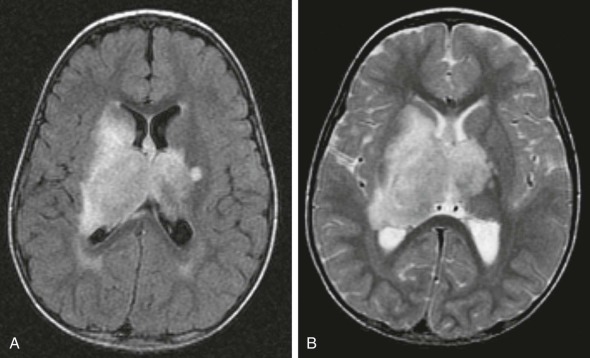
Management.
The presence of tumor in the thalamus presents a number of management difficulties. Because of their location, complete resection is difficult, although surgery can result in symptom improvement in selected cases. Even attempts at biopsy can result in significant morbidity. The small amount of surgical material often raises concerns of sampling error in attempting to determine the histopathologic grade of the tumor. Biopsy of the most malignant component of these deep-seated lesions can be guided with PET imaging. This guidance is particularly important for astrocytic tumors, for which samples of sufficient size and content are needed to define the elements of tumor grade. In the analysis of children with thalamic lesions, most tumors are unilateral in nature, and approximately 50% to 60% are low-grade astrocytomas; the remaining 40% are high-grade lesions. Some tumors are amenable to significant resection. The response of low-grade thalamic tumors to chemotherapy and radiation is not known to be different from that of similar tumors in other locations. Thus younger patients are often treated initially with chemotherapy used for other low-grade astrocytomas ( Table 57-5 ). In those with rapid progression or histologic verification of high-grade features, radiotherapy can be used, although a significant portion of the brain will receive a substantial dose, resulting in long-term toxicity for survivors.
Prognosis.
Contrary to some reports of very poor outcome for bithalamic astrocytomas of any grade, five of nine patients were long-term survivors in one retrospective series. Part of the difficulty in assigning an accurate prognosis to these tumors is the limited biopsy material available for assessment. With a number of reports of survivors now available, even in the context of bilateral disease, all young patients should be given a trial of chemotherapy.
Low-Grade Diencephalic Astrocytomas (Diencephalic Syndrome)
Diencephalic tumors remain a unique and poorly understood subtype of PLGGs. They typically occur in young infants, although a few patients have presented late in the first or second decade of life. A high incidence of dissemination throughout the neural axis has been recognized and may relate to the increased frequency of the pilomyxoid variant in this location. The presence of failure to thrive in infants often leads to an extensive and prolonged workup for gastrointestinal abnormalities before the correct cause is identified. The presence of diencephalic tumors with early age of onset and the propensity to disseminate suggest that unique biologic differences may exist among these tumors and other low-grade astrocytomas, although no specific abnormalities have yet been documented.
Clinical Presentation.
Diencephalic low-grade astrocytomas are differentiated from other LGGs in children by their presence around the hypothalamus–optic chiasm and a unique constellation of symptoms, including the three “E’s”—emaciation, emesis, and euphoria. In spite of severe failure to thrive that is often seen, most infants retain normal growth rates and maintain normal pituitary secretion prior to surgical resection. Although an accurate clinical picture for these patients continues to be defined, because many lack the typical constellation, their management is difficult because of the deep-seated nature of these lesions and the frequent presence of leptomeningeal dissemination at diagnosis. As such, these patients require full craniospinal imaging at diagnosis, along with CSF analysis. Any patient presenting with symptoms of spinal disease needs to undergo immediate restaging.
Imaging and Histology.
The imaging characteristics of diencephalic LGGs do not differ from those of low-grade astrocytomas in other locations. Lesions are centered around the hypothalamus and chiasm, are bright on T2-weighted images, and usually show homogeneous enhancement after administration of contrast material. The lesions are typically PAs or fibrillary astrocytomas, although the pilomyxoid variant can also be observed in this location. These tumors cannot be differentiated by mutational status of BRAF because some patients have the BRAF V600E mutation, some have the BRAF truncated fusion KIAA1549, and other patients have neither.
Management.
The approach to therapy follows that used for other low-grade astrocytomas. Because of the young patient age and deep location of these tumors, radiation therapy is usually contraindicated and will result in significant cognitive impairment over the long term. Most patients undergo maximal safe surgery, followed by chemotherapy with vincristine and carboplatin or TPCV (see Table 57-5 ). Radiographic response or stable disease accompanied by weight gain is common. High-dose chemotherapy has also been used successfully as an investigational approach. Radiographic responses and improvement is weight have also been reported with bevacizumab and irinotecan.
Prognosis.
In spite of the low-grade nature of diencephalic tumors, patients with these tumors do less well than do patients with similar tumors located in the optic pathway, brainstem, or cerebellum. In addition to the continued progression of these tumors, which leads to compression of vital structures, patients are at high risk for surgery-induced hypothalamic damage resulting in obesity.
Fibrillary Astrocytomas
Fibrillary (WHO grade II) astrocytomas are low-grade astrocytomas that are distinct from PAs. Precise determination of the incidence of fibrillary astrocytomas is difficult because many tumors, particularly those in deep structures of the diencephalon or brainstem, cannot be resected sufficiently to provide the material required to classify the tumor accurately. Thus the terms “fibrillary astrocytoma” and “low-grade astrocytoma” are often used interchangeably. Although all fibrillary astrocytomas are LGGs, most LGGs are PAs. Many tumors are classified as grade II astrocytoma with piloid features because a biopsy specimen may have been too small to identify all the elements required for classification as a grade I PA. Similarly the histologic term “pilocytic astrocytoma with fibrillary features” is used to indicate tumors that have all the components of grade I pilocytic astrocytomas but with areas of infiltration suggesting that they could be grade II. Other LGGs include pilomyxoid astrocytomas, oligodendrogliomas, and gangliogliomas. Fibrillary astrocytomas localized to the posterior fossa represent 3% to 15% of cerebellar tumors ; their incidence is lower in the remainder of the brain and spine. No gender predilection exists, and the peak age at diagnosis is 6 to 10 years. Genetic abnormalities in LGGs are uncommon. The reported incidence of LGGs has increased during the past several years. Although an association with paternal workplace exposure in the chemical and electrical industries is purported, a more likely explanation is the increased use and availability of MRI and the diagnosis of presymptomatic lesions.
Clinical Presentation.
The initial symptoms of fibrillary astrocytomas vary, depending on the location of the tumor. Patients with medullary tumors may present with a long history of dysphagia, hoarseness, ataxia, and hemiparesis. Cervicomedullary tumors may cause medullary or upper cervical symptoms such as neck discomfort, weakness or numbness of the hands, and an asymmetrical quadriparesis. Patients with midbrain tumors such as a tectal glioma often present with signs and symptoms of raised ICP. Other symptoms include diplopia and hemiparesis. In children with dorsally exophytic brainstem glioma, a component of the tumor arises in the medulla and expands in a dorsal direction, resulting in noncommunicating hydrocephalus. Supratentorial fibrillary astrocytomas more commonly present with seizures, although thalamic lesions present with motor deficits. Hypothalamic lesions can present as diencephalic syndrome. Low-grade astrocytomas in the brainstem are usually focal rather than diffuse. They tend to arise in the midbrain, cerebellar peduncles, medulla, or cervicomedullary region. Because of the slow rate of progression of these lesions, most patients have subtle neurologic changes that only become evident over a long period. Unlike adult fibrillary astrocytomas, with which degeneration to malignant gliomas is common, pediatric fibrillary astrocytomas remain low grade, even after multiple recurrences. Symptoms may exist for months to years prior to the diagnosis of a low-grade astrocytoma.
Imaging and Histology.
Most low-grade fibrillary astrocytomas appear isodense on CT without significant contrast enhancement. The tumors are hypointense on T1-weighted and hyperintense on T2-weighted MRI, with minimal or no gadolinium enhancement ( Fig. 57-23 ) except for dorsally exophytic brainstem tumors. This appearance is in contrast to that of most PAs, in which homogeneous contrast enhancement is common. Pathologic examination may demonstrate some cellular pleomorphism, but no mitoses, necrosis, or endothelial proliferation are present (i.e., no histologic features of malignancy are present). Although PAs are usually well-circumscribed lesions with a biphasic pattern of areas of bipolar cells and Rosenthal fibers and other areas with microcysts, fibrillary astrocytomas have greater cellularity and infiltrating boundaries. In contrast to grade III or IV astrocytomas, fibrillary astrocytomas must lack features of malignancy, such as significant atypia and pleomorphism, mitoses, vascular proliferation, and palisading necrosis. A number of other subtypes of grade II astrocytomas have been identified, including the lipoastrocytic, protoplasmic, gemistocytic, and xanthomatous types. These subtypes are much less common in pediatric patients compared with adults, and may have a worse outcome compared with fibrillary astrocytomas and PAs. The rarity of these lesions prevents formal studies of these subtypes.
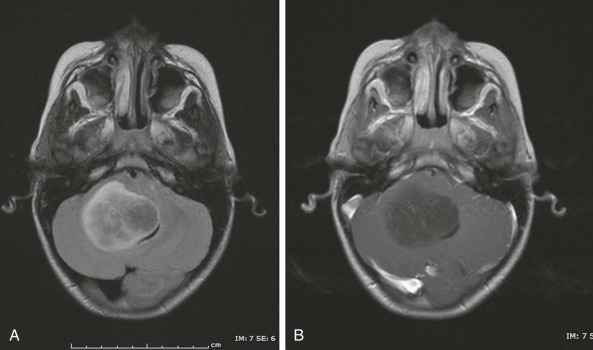
Management.
Management of most fibrillary astrocytomas is similar to that of PAs and depends on the clinical prodrome, age, and location of the primary tumor. Rapidly evolving clinical symptoms in the setting of an operable tumor usually warrant prompt neurosurgical intervention. On the other hand, a lesion with a long history of indolent and mild symptoms is often managed with close MRI and clinical surveillance. In patients who subsequently show progressive neurologic symptoms and for whom MRI studies suggest tumor growth, therapeutic intervention is required. Diffuse fibrillary astrocytomas in eloquent locations such as the thalamus or motor regions are often biopsied to confirm the diagnosis and to exclude higher grade glial tumors. They do not lend themselves to radical resections, as is commonly performed for PAs. However in certain cases, such as dorsally exophytic brainstem astrocytomas, radical resection can often confer a long symptom-free outcome. In cases in which resection is not feasible, chemotherapy or radiotherapy may be indicated. As in the case of progressive or unresectable PAs, chemotherapy is the preferred first approach for patients (see Table 57-5 ). Patients respond well to either vincristine and carboplatin or TPCV chemotherapy. A recent randomized clinical trial of grades I and II astrocytomas has been completed in which these two treatment regimens were compared. Although recurrences occur in most patients, necessitating retreatment with other chemotherapeutic agents, the overall prognosis remains good. Experience with novel combinations of agents or high-dose chemotherapy is limited. Radiotherapy is reserved for older children or younger patients whose tumors progress and are refractory to chemotherapy. Reduced radiation doses to adjacent normal tissue, with equivalent tumor control to that of standard photon therapy, can be achieved with conformal proton therapy. Radiation therapy delivered at the time of initial diagnosis does not appear to provide additional benefit for OS.
Prognosis.
The long-term survival rate for completely resected pediatric supratentorial low-grade astrocytomas is excellent, especially when compared with similar tumors in adults. Even partially or unresected fibrillary astrocytomas may remain stable for many years. An example of this is the focal midbrain or tectal glioma, which is rarely biopsied or resected. Response to chemotherapy appears to be similar to that observed in PAs, although most patients will experience one or more episodes of progression, requiring retreatment with additional chemotherapy. However children with primary tumors arising in the pons or thalamus have a worse prognosis. The presence of a gemistocytic component characterized by a predominance of large astrocytes, with thick processes and dramatic accumulation of GFAP, represents a histologic variant of low-grade fibrillary (grade II) astrocytoma. This variant may be associated with p53 mutations and may also convey a higher predisposition to malignant transformation.
Although most patients with low-grade astrocytomas will survive, patients with fibrillary astrocytomas have a poorer outcome than patients with grade I astrocytomas. Because PAs are more focal and easier to resect than are fibrillary astrocytomas, prognosis may be related to the ease of resection rather than to biologic differences between these histologic variants. Overall survival after chemotherapy of children and adolescents with low-grade astrocytomas at 10 years is 70% to 80%. The role of MIB1 expression in the prognosis of low-grade astrocytomas is not established as a prognostic factor, after age and degree of resection are excluded. Survivors of low-grade astrocytomas still have a number of limitations. Future treatment efforts for these tumors therefore will need to combine effective therapy with improved quality of survivorship in these patients.
Tectal Gliomas
Tectal gliomas are typically hamartomas or low-grade astrocytomas, and patients present with acute hydrocephalus in most cases. These tumors appear to represent a unique variant of low-grade tumors, based on their positive long-term outcome, with relief of hydrocephalus as the sole therapeutic intervention. Because biopsy or surgical resection of these lesions is rarely required, little is known about the pathways that drive their activation. No known genetic syndromes give rise to these tumors.
Presentation.
Most patients with tectal gliomas present with the symptoms of obstructive hydrocephalus because of expansion of these lesions adjacent to the periaqueductal space. When the presence of these tumors is picked up incidentally, many patients will demonstrate prolonged periods of stability and do not require treatment.
Imaging and Histology.
The radiographic appearance of most tectal gliomas is similar to that of other LGGs. Most tumors lack contrast enhancement ( Fig. 57-24 ). Based on their location and presenting symptoms, histologic verification is not required to make the diagnosis and can cause significant morbidity if attempted.
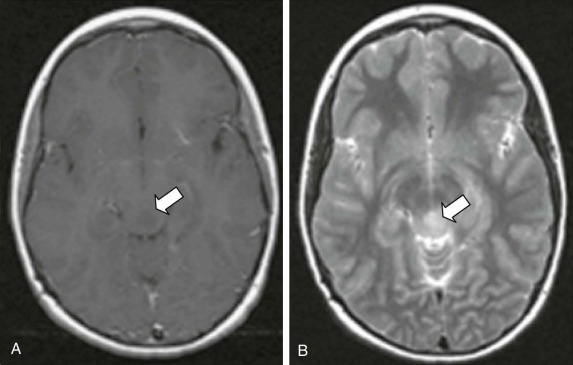
Management.
Biopsy or resection of tectal gliomas is usually not required. Rather, most patients require immediate CSF diversion through a third ventriculostomy. Failures of third ventriculostomies have been reported, even many years after the initial procedure, necessitating the proper education of families regarding symptoms that should precipitate immediate evaluation. Reduction in ventricular size is often incomplete and the ventricular size continues to change during the first year after diversion. Rarely tectal gliomas larger than 10 cm at presentation will continue to progress over time and may require surgical debulking and chemotherapy. Tumors that have atypical radiographic features, rapid progression, or repeated recurrence may require a biopsy. Tumors with asymptomatic progression can often be observed carefully. When treatment is required, approaches similar to those used for other low-grade astrocytomas are recommended. Chemotherapy is usually initiated. Radiation therapy is not required for most patients, and continued progression of these lesions into adulthood is rare.
Prognosis.
The long-term outcome for patients with tectal gliomas remains excellent, which is surprising given the unresectable nature of the lesion and the absence of therapy for most patients. Overall survival approaches 100% in this population, and thus avoidance of unnecessary surgical or radiation-related long-term morbidity is critical.
Pilomyxoid Astrocytomas
Pilomyxoid astrocytomas are a newly stratified group of grade II pediatric tumors in the 2007 WHO classification that were previously grouped together with PAs. Although no specific molecular pathway responsible for these tumors has yet been identified, the presence of defects in the BCR gene on chromosome 17 provides possible clues that require further analysis. Other chromosomal aberrations have also been identified. Pilomyxoid astrocytomas are found most commonly in the midline of the brain and spine. These tumors are usually identified in infants and young children, although their presence in adults has been reported. At recurrence, tumors can appear as classic PAs, suggesting a developmental relationship between the two types of tumors.
Clinical Presentation.
Although pilomyxoid astrocytomas present in a manner similar to that of other LGGs, the incidence of metastatic disease at presentation appears higher and requires a full workup, including spinal MRI and CSF for cytologic evaluation. Patients with pilomyxoid astrocytomas may be at greater risk for spontaneous hemorrhage at the time of diagnosis or resection or during follow-up.
Imaging and Histology.
On MRI pilomyxoid astrocytomas appear similar to PAs, with well-circumscribed margins and little peritumoral edema. They are usually found in the midline, including the brainstem and spine, and can be solid or solid and cystic. They are usually bright on T1- or T2-weighted and FLAIR sequences, and they enhance with administration of contrast material. In approximately 50% of cases, the contrast enhancement is heterogeneous. Adjacent areas of brain can demonstrate elevated choline-to-creatinine ratios suggestive of an infiltrative margin. Although their location, young patient age, and increased presence of dissemination provide clues to the diagnosis before a biopsy is performed, imaging characteristics do not clearly differentiate these tumors from other LGGs in the pediatric population. Histologically these lesions lack many features of PAs, such as Rosenthal fibers and a biphasic pattern. Rather a monophasic pattern and myxoid background is seen, with strong GFAP and synaptophysin staining. Like other glial tumors, mixed lineages may also be possible for pilomyxoid astrocytoma. The variable histologic appearance compared with PAs, MRS signal changes, and a higher incidence of progression and dissemination support their distinction from PAs. A predilection for younger age appears to exist when the tumor is localized to the pituitary-hypothalamic region.
Management.
Because of the more aggressive nature of these tumors compared with PAs, pilomyxoid astrocytomas are now classified as grade II gliomas. Treatments typically follow those of other LGGs, including vincristine and carboplatin and TPCV chemotherapy. Other therapies used for PLGGs have also been used for patients with pilomyxoid astrocytomas ( Table 57-5 ). Upfront chemoradiation therapy combinations have also been tried for these tumors. Formal clinical trials for this rare subtype are not possible, however. Because of the young age of the patient and the deep location of these tumors in many patients, radiation-based strategies will lead to significant long-term morbidity in most survivors. Many patients with pilomyxoid astrocytomas are infants and have diencephalic syndrome with dissemination, and thus complete surgical resection is not possible. Thus most patients will start with LGG chemotherapy. The use of intrathecal therapy for patients with disseminated disease who are unable to receive craniospinal radiation can be considered.
Prognosis.
Without well-defined studies, the prognosis of pilomyxoid astrocytoma is difficult to assess with certainty. Confounding issues of deep-seated lesions, especially around the hypothalamic region in which surgical options are limited, along with the young age of these patients and the presence of metastatic disease, likely affect the overall poor prognosis. A mean OS of 60 months for patients with pilomyxoid astrocytoma versus 220 months for patients with grade I PAs has been reported. Whether their unique biology affects survival is unknown, although these tumors can recur as typical grade I pilocytic astrocytomas. As in other LGGs, nestin expression may correlate with a worse prognosis.
Ganglioglioma and Glial-Neuronal Tumors
Gangliogliomas are low-grade (WHO grade I) glioneuronal tumors. These tumors represent 4% to 8% of primary brain tumors in children, with 80% occurring before age 30 years and at a mean age less than 10 years. These tumors are frequently associated with seizures and tend to grow slowly. Gangliogliomas can occur throughout the CNS, although most are localized to the temporal and parietal lobes. A significant proportion of these tumors have the BRAF V600E mutation, which is now being targeted in open international clinical trials. The presence of this oncogenic mutation may result is a more aggressive behavior and worse outcome.
Clinical Presentation.
Seizures are the first manifestation in 50% of cases of ganglioglioma, and many patients have a prolonged history of seizures of longer than 2 years. Complex partial seizures are common because gangliogliomas are frequently located in the temporal lobe, particularly the temporomesial region, although they can occur anywhere in the brain or spine.
Imaging and Histology.
Gangliogliomas are typically contrast-enhancing cystic lesions on CT scans. The MRI appearance of these tumors can be variable but is frequently hypointense on T1-weighted sequences and hyperintense on T2-weighted images. Gadolinium enhancement varies in intensity from absent to significant and can be nodular, solid, or circumferential. An infantile variant of ganglioglioma, desmoplastic infantile ganglioglioma (DIG), can occur (discussed later). Pathologic studies show synaptophysin and neuronal nuclear antigen (NeuN)–positive ganglion cells, as well as a GFAP-positive astrocytic component. Lesions associated with the ganglion cell but lacking the astrocytic component are called gangliocytomas. Gangliogliomas are frequently WHO grade I tumors, although some may show anaplastic features in the glial or neural component. MIB1-positive cells are usually localized to the astrocytic component. Glial-neuronal tumors likely represent a spectrum of LGGs that include gangliogliomas and may also have PI3K pathway activation.
Management.
Complete or near-total resection is the treatment of choice and, in most cases, can eliminate or significantly improve seizure frequency in this patient population. Recurrent or unresectable tumors may be treated with radiation therapy, although these lesions may respond to LGG chemotherapy, and thus patients may avoid some of the long-term toxicity of radiation. In the case of recurrent or unresectable ganglioglioma, response to LGG chemotherapy has been demonstrated. Treatment for recurrent or unresectable gangliogliomas is included in current LGG chemotherapy trials. It should be noted that these tumors have been shown to undergo malignant transformation over time, usually involving the astrocytic component. Clinical trials of BRAF V600E inhibitors are under way for persons with this mutation, and significant durable responses have been reported.
Prognosis.
GTR is often curative, making tumor location and extent of resection the most important prognostic factors. Most patients are rendered seizure-free after GTR. Gangliogliomas of the posterior fossa have also been reported, and their outcomes appear to be similar to those for supratentorial lesions. Patients whose tumors can be fully resected to remove the enhancing components are likely to remain disease free, although persons with subtotal resected disease often require additional resection, chemotherapy, and/or radiation therapy. The uncommon presentation of these tumors in the infratentorial location and the limited sample sizes in case reports limit the confidence with which therapeutic approaches can be recommended. Spinal cord ganglioglioma appear to have an indolent course. Patients with subtotal resection can remain stable for prolonged periods. Therefore treatment should be deferred until lesions show clear evidence of progressive disease. Very rarely, gangliomas and glial neuronal tumors can degenerate into a CNS PNET (the neural component) or into a malignant glioma (the astrocytic component).
Desmoplastic Infantile Gangliogliomas
DIGs are supratentorial tumors involving the leptomeningeal surface and are identified predominantly in children younger than 2 years, although reports in older children are available. These lesions are often very large, in part because of the presence of cysts, and they frequently involve the dura. Although precise estimates of their incidence are lacking, they make up fewer than 1% of pediatric brain tumors. A number of chromosomal abnormalities have been reported, although only in a small number of cases, particularly at chromosome 7q31 (corresponding to MET gene). One case containing the BRAF V600E mutation was also noted in this series and was reported in two other patients in a different analysis.
Clinical Presentation.
Most patients present with increasing head circumference, bulging fontanelle, and lethargy. Older patients present with focal motor deficits.
Imaging and Histology.
DIGs are large cystic structures on CT scans, with contrast enhancement of the solid component. T1-weighted MRI sequences demonstrate an isointense tumor; T2-weighted intensity of the tumor is variable. The tumors usually enhance after administration of contrast material. Peritumoral edema is uncommon. DIGs possess a desmoplastic stromal background, with neoplastic neurons and astrocytes. They often have areas with an elevated MIB1 labeling index, although this characteristic does not usually represent transformation to a more malignant phenotype. Tumors lacking the neural component are called desmoplastic infantile astrocytomas.
Management.
Cure can be achieved with complete resection. Many patients have remained progression-free after total resection without additional treatment. Chemotherapy, as for other low-grade astrocytomas, can be considered for patients with symptomatic or progressive cases for whom surgical removal is not feasible.
Prognosis.
Most patients will be long-term survivors if a complete resection is achieved. Lesions that are more deep-seated may have a poorer outcome, and initiation of chemotherapy may be considered at the first signs of progression in these unresectable tumors. Metastatic lesions have been reported, suggesting that in rare cases, a more malignant phenotype can occur.
Dysembryoplastic Neuroepithelial Tumors
DNETs are recently described tumors that may comprise as many as 1% of all brain tumors in patients younger than 20 years. Two thirds of all DNETs are located in the temporal lobe, and DNETs are found in 5% to 15% of temporal lobe resections for intractable epilepsy. These lesions are classified as WHO grade I tumors. Thought to be developmental in nature, they are treated with surgery. They are considered to have limited proliferative potential, suggesting that complete surgical resection is not required for long-term disease control ; nonetheless, recurrences can occur.
Clinical Presentation.
The diagnosis of DNET should be a consideration in children and young adults with new-onset seizures or a long history of epilepsy. Patients with these tumors typically present with a long history of complex partial seizures, with an average age of onset of 9 years. In many patients the seizures are refractory to anticonvulsant medications. The superficial cortical location of DNETs may account for the high risk of seizures.
Imaging and Histology.
Contrast-enhanced cranial MRI typically shows a temporal or frontal lobe lesion, absent peritumoral edema, and only minimal, if any, gadolinium enhancement. The tumors are bright on T2-weighted sequences and hypointense on T1-weighted images ( Fig. 57-25 ). Mass effect is minimal to absent. Although routine fluorine 18–labeled deoxyglucose–PET imaging has not been helpful in the preoperative identification of these lesions, Carbon 11–methionine–PET was able to distinguish these lesions from other low-grade lesions. The pathologic findings include a specific glioneuronal element manifested by GFAP-negative oligodendroglia-like cells and neurons in a mucinous eosinophilic background that give the appearance of floating neurons. Because oligodendrocytes, astrocytes, or both are found on histopathologic analysis, the pathologic differential diagnosis often includes oligodendroglioma, mixed oligoastrocytoma (OA), and ganglioglioma. Like gangliogliomas and PXAs, DNETs have been reported to have the BRAF V600E mutation in approximately 30% of cases. Differentiation of DNETs from other LGGs can be difficult.
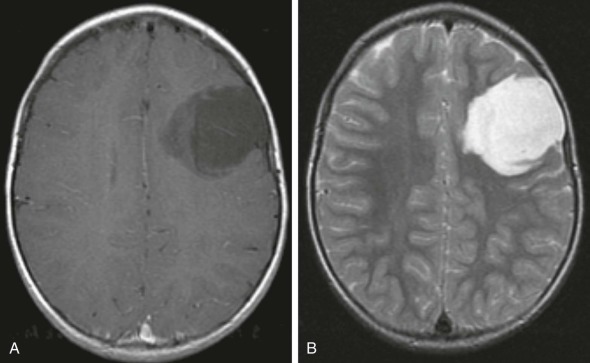
Management.
Although these tumors have a benign course, the associated seizures may be refractory to antiepileptic drugs because of the increased expression of multidrug transporters. Gross total resection is often curative and typically alleviates the seizures, especially if the areas of cortical dysplasia can also be removed, although some case series have shown a significant number of patients who continue to have residual seizures. Adjuvant chemotherapy or radiation therapy is not recommended. Rare cases of malignant transformation of DNETs after radiation and chemotherapy have been reported.
Prognosis.
The stable behavior of these tumors over time results in an excellent prognosis after gross total or partial resection. Patients often have improved seizure control after surgery, especially if a complete resection was achieved, and some improvement in neuropsychologic functioning has been observed.
Pleomorphic Xanthoastrocytomas
Pleomorphic xanthroastrocytomas (PXAs) are uncommon grade II cortical tumors that mainly occur in children and adolescents and account for fewer than 1% of CNS tumors. The median age at the time of diagnosis is 14 years. The molecular pathogenesis of these tumors is poorly understood, and no causative chromosomal abnormalities have been identified. The BRAF V600E activating mutation has been detected in more than 50% of cases, and drugs targeting this mutation are now in clinical trials. A recent analysis of 50 PXAs by comparative genomic hybridization, however, has revealed that loss of chromosome 9 is the most common chromosomal lesion. A few reports of PXA in conjunction with NF-1 may implicate the Ras pathway in this disease.
Clinical Presentation.
PXAs are typically large and superficially located, especially in the temporal lobes. Seizures are the most common presenting feature. For tumors of the cerebellum, pineal region, or spine, direct nerve compression results in focal deficits, and obstruction of CSF flow results in raised ICP. Rare cases of dissemination have been reported.
Imaging and Histology.
PXAs typically manifest enhancement on MRI, with occasional intratumoral cysts and calcification ( Fig. 57-26 ). Peritumoral edema is uncommon. Tumors usually extend to the meninges. The typical histopathologic picture includes a pleomorphic appearance of the astrocytic component, with significant cellular atypia and bizarre multinucleated giant cells with intracellular lipid accumulation. The proliferative indices are usually low, although necrosis, endothelial proliferation, and mitoses have been described. PXA can be confused with glioblastoma multiforme because of the presence of multinucleated cells and occasional foci of necrosis. The expression of CD34 on most tumors may be useful in the histologic classification of difficult or atypical cases. Prognosis appears to correlate with MIB1 expression. In lesions with mitoses, an elevated MIB1 labeling index, and necrosis, the term “pleomorphic xanthoastrocytoma with anaplastic features” is used.
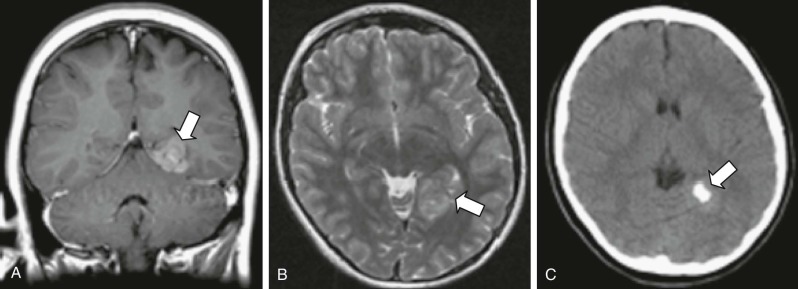
Management.
The clinical diagnosis of PXA should be suspected in children who present with new-onset seizures, focal deficits, and a large, enhancing cortical mass on brain imaging. The goal of surgery is to achieve a GTR, which is usually curative. Adjuvant therapy can be deferred, although the patient should be followed expectantly. Tumors that recur and incompletely resected lesions with anaplastic features may be treated with chemotherapy or radiotherapy, although significant activity for these modalities has not been demonstrated. Few formal clinical trials for this rare subtype have been performed.
Prognosis.
Several series have reported a 5-year PFS of greater than 70%. However the presence of mitoses, endothelial proliferation, or necrosis on the pathologic specimen, although rare, may significantly alter the clinical behavior and prognosis. Cases of anaplastic PXA with subsequent malignant transformation have been reported. Radiation therapy does not alter the poor outcome in these cases.
Subependymal Giant Cell Astrocytomas
SEGAs usually originate in the ependymal walls of the lateral ventricles and are associated almost exclusively with TS, an autosomal-dominant disorder. Patients with TS have hamartomas and benign tumors of multiple organs, including the brain. The CNS manifestations include cortical tubers (hamartomas), subcortical glioneuronal hamartomas, subependymal glial nodules, and subependymal giant cell astrocytomas. Two genetic defects, one in the TSC1 gene on chromosome 9q and the other in the TSC2 gene on chromosome 16p, account for TS. Sporadic cases are rare.
Clinical Presentation.
SEGA is sometimes the presenting feature of TS in patients without the typical physical stigmata of the syndrome. Because of the variable phenotype of TS, a consensus on diagnostic criteria has been developed. Presentation in older children and adolescents is common. Although patients with TS typically have multiple periventricular SEGAs, those that produce symptoms of hydrocephalus arise in close proximity to the foramen of Monro, although lesions around the pineal gland can also result in symptoms. Most patients with known TS are followed up with regular neuroimaging and the SEGAs are removed serious neurologic syndromes, such as headaches, altered sensorium, or weakness are present. Of note, patients with TS may have other significant neurologic symptoms, such as seizures and cognitive deficiency related to cortical tubers.
Imaging and Histology.
CT and MRI are essential for early and accurate diagnosis. Although CT is superior to MRI for detecting small calcified lesions, MRI is superior to CT for identifying areas of gliosis, heterotopia, and SEGAs, which have the typical radiographic appearance of candle dripping. SEGAs typically show diffuse contrast enhancement on CT and MRI studies ( Fig. 57-27 ). SEGAs are considered grade I astrocytomas. They are well-circumscribed tumors that are positive for GFAP, neurofilament, neuron-specific enolase, and synaptophysin. Mitoses can be identified but do not indicate a higher grade or more malignant phenotype. They can occasionally be associated with bleeding.

Management.
The management of patients with TS is complex and requires adherence to recent consensus conference guidelines. GTR is the treatment of choice for SEGAs that are progressive, and cause obstructive hydrocephalus or seizures. The major risk of surgery is injury to the forniceal columns, resulting in memory disturbance. The benign behavior of these tumors warrants reoperation in the case of recurrence or progression after subtotal resection. Inhibition of the mTOR pathway in SEGAs has resulted in a very high response rate, and mTOR inhibitors have now been approved for the treatment of these lesions.
Prognosis.
SEGAs are essentially benign tumors, and gross total resection is generally curative. However multiple tumors may arise in some patients with TS, requiring ongoing radiologic surveillance. The overall prognosis for patients with TS is good, despite increased susceptibility to other tumor types, including rhabdomyomas of the myocardium and angiomyomas of the kidney, liver, adrenals, and pancreas.
Hemangioblastomas
Hemangioblastomas are WHO grade I lesions associated with von Hippel-Lindau (VHL) disease. They occur primarily in adults and are centered in the cerebellum, brainstem, and spinal cord. Although sporadic tumors can arise, pediatric cases are usually associated with the inherited syndrome. Cases associated with VHL disease can be single or multifocal, although most sporadic cases involve isolated nodules. Hemangioblastomas are highly vascularized lesions, although the genetic defect is localized to the stromal elements rather than the vasculature. Tumor development appears to be related to the deregulation of VEGF. Patients with hemangioblastomas need to undergo complete genetic evaluation for VHL disease and, for those with the inherited disease, careful lifelong screening is necessary.
Clinical Presentation.
Obstruction of CSF flow is the primary reason for symptomatology in patients with VHL disease who are diagnosed with hemangioblastomas. Headaches, morning vomiting, and long tract signs are therefore common. Ataxia and cranial nerve dysfunction can also be observed.
Imaging and Histology.
Hemangioblastomas are typically well-circumscribed cystic lesions with a solid tumor nodule. The solid component tends to enhance brightly with contrast and is usually located peripherally within the cyst ( Fig. 57-28 ). Flow voids are commonly observed on MRI and can be well delineated with angiography. Peritumoral edema is commonly observed. Tumors are usually composed of two elements—the stromal cells, which express vimentin and VEGF, and the endothelial cells, which express the VEGF receptor VEGFR2. Hemangioblastomas typically have low proliferative levels based on MIB1 immunoreactivity and are considered grade I tumors.
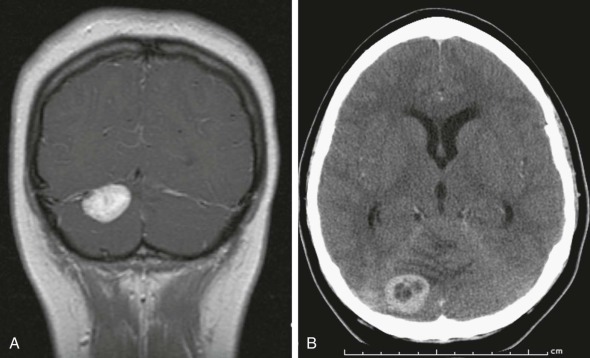
Management.
The management of these tumors requires specialized neurosurgical expertise because of the high risk of bleeding. The primary approach to these lesions, which tend to occur most commonly in the posterior fossa and cervicomedullary area, is maximal surgical resection. This approach provides long-term control in most patients, although, because of the underlying genetic predisposition, new tumors may continue to develop throughout the life of the patient. Embolization can reduce the intraoperative risks of bleeding. Radiosurgery can successfully treat the mural component of these tumors, although control is negatively influenced by the presence of the cysts. VEGF inhibitors have been tested in this population with initial encouraging results, although follow-up of these reports is still pending. A case of prolonged stable disease with thalidomide treatment was reported, although whether this outcome was related to its antiangiogenic or immune modulatory effects is unknown.
Prognosis.
Because of the genetic basis of these lesions, recurrences are common and likely represent new tumors rather than recurrences of previously resected ones, which indicates the importance of continued careful evaluation in this patient population.
Rare LGGs
Tanycytic Astrocytomas.
Tanycytic astrocytomas are rare and poorly characterized tumors. They are most common in adults and usually occur in the hypothalamic region. Their malignant potential is not well defined, although recurrences after complete resection have been observed. One reported case occurred in the context of an adolescent with NF-2.
Lipoastrocytomas.
Lipoastrocytomas are rare cortical tumors that, as the name suggests, possess fat droplets that give them the appearance of adipocytes in the context of a low-grade astrocytic lesion. These tumors express GFAP. Recurrences can occur and are treated with resection. Malignant transformation has not been reported in this tumor type, although one case was identified on autopsy in a patient with diffuse intrinsic pontine glioma.
Dysplastic Cerebellar Gangliocytomas.
Dysplastic cerebellar gangliocytomas are rare tumors of the cerebellum and fourth ventricle region that are of limited proliferative potential. They have a characteristic imaging pattern on MRI, with an abnormal laminated pattern in the cerebellum, although this pattern is not pathognomonic and can be mimicked by medulloblastoma. As such biopsy is recommended to differentiate the two entities. Dysplastic cerebellar gangliocytomas do not typically require therapeutic intervention, although seizure activity can occur as a result of their presence.
Disseminated Oligodendroglial-Like Leptomeningeal Tumor of Childhood.
Disseminated oligodendroglial-like leptomeningeal tumor of childhood is a newly described entity that typically demonstrate leptomeningeal enhancement on MRI and associated cystic or nodular T2 hyperintense lesions within the spinal cord/brain along the subpial surface. A discrete intraparenchymal lesion, usually in the spinal cord, is found in the majority of cases. These tumors are thought to have a greater risk of malignant transformation.
High-Grade Astrocytomas
High-grade astrocytomas (HGAs) encompass anaplastic astrocytoma (WHO grade III) and glioblastoma (previously glioblastoma multiforme, WHO grade IV) of the brain and spinal cord. This category also includes as a subgroup DIPG, even though these tumors occasionally show lower grade histology. HGAs, unlike LGGs, are much less common in children than adults. Whereas more than 30% of all pediatric brain tumors are LGGs, non-DIPG HGAs represent only 6% to 12% of all primary pediatric brain tumors, and DIPGs represent another 10%. In the past several years, many key discoveries have been made about the biology of these tumors, and increasingly, important distinctions from adult HGA biology are being found. Unfortunately major treatment advances have not yet followed the biologic discoveries, and most children with these tumors eventually succumb to their disease. Because of key differences between DIPGs and other pediatric HGAs, most notably in prognosis and management, DIPG is discussed separately from the remainder of tumors in this category.
Supratentorial HGAs (Excluding DIPG).
Although adult and pediatric HGAs are similar histologically, increasing evidence indicates key biologic differences, even within shared pathways of tumorigenesis. Common pathways include the overexpression or mutation of EGFR, although both changes are less frequent in pediatrics, activation of the PI3K signaling pathway via silencing of the PTEN tumor suppressor, most often by methylation in pediatrics, and the MGMT DNA repair pathway, which is commonly methylated in adult HGAs but is more often overexpressed in pediatric tumors, perhaps helping to explain TMZ resistance in children with HGAs.
Recent findings have also demonstrated involvement of new pathways and features of pediatric HGA, further distinguishing them from adult tumors. These new pathways include overexpression of PDGFRα and novel histone mutations in H3F3A (observed in a minority of these tumors vs. a majority of DIPGs). Comprehensive profiling of HGA genetics has shown gains in chromosome 1q in pediatric HGA on copy number analyses, which is unusual in adults; fewer cases show chromosome 7 gain and 10q loss, two features seen in the majority of adult HGAs. One study appeared to show two distinct gene expression profiles in pediatric HGA, one that is similar to adult HGA, and one that is divergent. The PARP DNA repair pathway is also overexpressed in many pediatric HGAs, and this feature is associated with a poor prognosis ; PARP inhibitors may be useful as radiosensitizers. Aurora kinase B is overexpressed in a majority of pediatric HGAs. Unlike in adults, IDH1 mutations have not been found in pediatric HGAs. Pediatric tumors have demonstrated abnormalities in the Ras and Akt pathways, as well as Y-box protein-1. Microsatellite instability, which has been associated with a number of adult tumors, is very rare in pediatric HGAs.
Overexpression of many proteins distinguish pediatric HGAs from LGGs, including the DNA repair protein insulin-like growth factor binding protein 2 (IGFBP2), the cancer-testis antigen and possible immunotherapy target NY-ESO-1, the p53 inhibitor HDMX, the angiogenic marker endoglin, and the catalytic subunit of human telomerase reverse transcriptase (HTERT) ; the differentiation marker NF1A is underexpressed in pediatric HGA compared with LGG. In the CCG study of children with malignant HGGs (CCG945), p53 status was an important independent prognostic variable, with a 5-year PFS of 44% in tumors expressing a low level of p53 versus 17% in those overexpressing p53. Finally new epigenetic findings in pediatric HGAs include hypermethylation of the LIM homeobox 9 (LHX9) transcription factor, downregulation of class II and IV histone deacetylases (HDACs), acetylation of histone H3.3, and a decrease in adenosine deaminase, ribonucleic acid (RNA)-specific, 2 (ADAR2)–mediated RNA editing.
Clinical Presentation.
The clinical manifestations of HGAs depend on the anatomic location and the age of the patient. The clinical prodrome is usually short and rapidly evolving, with signs and symptoms of elevated ICP and focal neurologic deficits. A protracted course may evolve in the usual case of malignant transformation of a low-grade glioma, but in general, transformation of low-grade gliomas to high-grade gliomas is extremely rare in children, unlike in adults. Dissemination of HGA into the cerebrospinal fluid is less common than for medulloblastoma and other neural tumors but is being recognized more frequently, particularly as patients survive longer. Approximately 3% of pediatric patients with an HGA will have CNS metastatic disease at presentation.
Imaging and Histology.
Typical HGAs have an MRI appearance of heterogeneous enhancement or diffuse nonenhancing tumor with significant edema on the T1-weighted image, compressing or displacing adjacent ventricular structures and occasionally causing hydrocephalus. The T2-weighted signal is often more diffuse, consistent with both infiltrative tumor and edema ( Fig. 57-29 ). MRS demonstrates a markedly elevated choline-to-NAA ratio; a 40% increase in choline over normal brain tissue indicates an HGA with 90% sensitivity and specificity. Lesions tend to be 18 F-labeled deoxyglucose–PET avid, although F-fluoro-ethyl-tyrosine (FET)–PET may be superior for treatment planning and distinguishing between LGGs and HGAs. Technetium 99m–sestamibi SPECT (MIBI SPECT) imaging can also detect HGAs and can be used to follow up for response or recurrence, as can serial diffusion weighted assessment. Areas of hemorrhage within pediatric HGAs at diagnosis are not uncommon. The presence of an HGA within the volume of a previously irradiated field is difficult to differentiate from a radiation-induced high-grade transformation of a low-grade astrocytoma, although preliminary studies have identified some molecular differences distinguishing radiation-induced glioblastoma. Malignant features on histopathologic examination of HGA include nuclear pleomorphism, mitoses, palisading necrosis ( Fig. 57-30 ), endothelial proliferation ( Fig. 57-31 ), and a high Ki-67 or MIB1 labeling index (see Fig. 57-17 ), although high interobserver variability exists between pathologists in judging these features. Most pediatric HGAs are nestin-positive on immunohistochemistry. Distinguishing between grade III and IV tumors relies on differences in histologic features; all HGAs must demonstrate nuclear atypia and mitoses, whereas grade IV tumors must also show either endothelial proliferation, necrosis, or both. It has now been shown that a proportion of CD133+ HGA cells costain for CD105 (endoglin), an angiogenesis marker, suggesting that vasculogenic mimicry occurs in pediatric HGAs, which may help increase angiogenesis in HGA through the formation of fluid-conducting vessels of tumor cells. CD105 staining is an unfavorable prognostic marker.
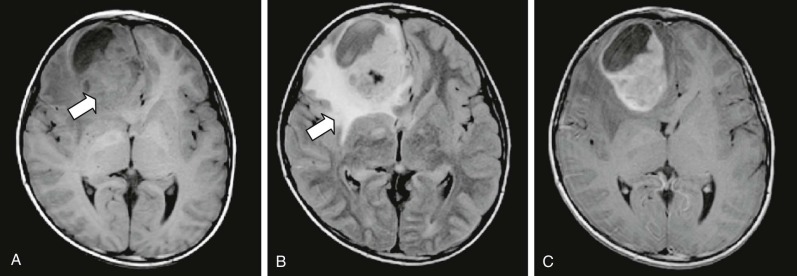
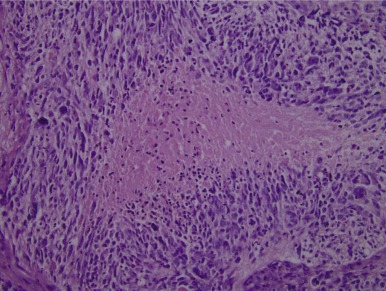
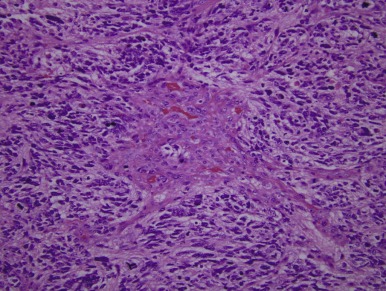
Management.
The initial management of pediatric HGA after neuroimaging is to administer high-dose corticosteroids to achieve symptomatic relief. Subsequently the goals of neurosurgery include establishing a histologic diagnosis and, whenever possible, achieving GTR. GTR is often not possible given the infiltrative properties of HGA, but even subtotal resection that achieves internal decompression or debulking makes subsequent radiotherapy more tolerable and probably more effective, given the lower tumor burden. Resection also diminishes the duration of corticosteroid therapy. Intraoperative MRI appears to increase the likelihood of GTR. The role of radiation therapy in the treatment of older children has been clearly established during the past 30 years, and the current standard in patients 3 years of age and older is a conformal dose of 5400 cGy to the primary tumor volume with a boost to 5940 cGy in the tumor bed.
Ever since the first national randomized trial comparing radiation versus radiation with chemotherapy in pediatric HGAs (CCG-943) showed a survival benefit for patients treated with chemotherapy, the use of adjuvant chemotherapy has been standard in the treatment of pediatric HGAs. However subsequent trials have failed to show a similar benefit, and the role of chemotherapy in the disease remains unclear. One area of recent focus has been the use of TMZ, which has become the standard of care in adult HGA. However a primary pediatric HGA phase II trial of concomitant TMZ and radiation therapy, followed by adjuvant TMZ, failed to show an improvement in overall or EFS compared with historic control subjects. Other phase II trials of the mTOR inhibitor temsirolimus, the VEGF inhibitor bevacizumab, and TMZ in combination with the DNA repair inhibitor O6-benzylguanine, all in recurrent or progressive pediatric HGA, also failed to meet their aims. Radiosensitizers, including inhaled carbogen, have also failed to show efficacy. A strategy that has shown potentially more promise is multiagent chemotherapy, with or without ASCR. Multiagent, multicourse chemotherapy with radiation therapy improved survival over historic control subjects in patients with primary HGA who had undergone GTR. Another trial of multiagent chemotherapy given over a long period showed promise in delaying radiation therapy for patients with HGA who were younger than 3 years, given the especially severe neurodevelopmental effects of radiation in this age group. Treatment with high-dose chemotherapy and ASCR has also been attempted in this group. The risk-benefit ratio is still under investigation, with initial reports of a 23% overall response but a mortality rate of 16%. A trial of high-dose thiotepa and carboplatin followed by ASCR produced one long-term survivor out of four children treated for recurrent or progressive HGA; remission at the time of treatment appeared to be a key positive prognostic factor in this trial, which also included children with high-grade nonastrocytic brain tumors.
Clearly, further study of the role of chemotherapy in pediatric HGAs is needed, as are new strategies. A current COG trial is comparing three potential chemotherapy regimens (the HDAC inhibitor vorinostat, TMZ, and bevacizumab) to be given with radiation therapy, followed by maintenance chemotherapy with TMZ and bevacizumab. Recent phase I trials have used the EGFR inhibitor erlotinib and a prolonged dosing schedule of TMZ. An autologous dendritic cell–based vaccine has shown potential efficacy. Oral valproic acid has shown some effect retrospectively and is well tolerated, making it an attractive potential additive agent for future trials. New mouse models of pediatric HGA inspire hope for more effective preclinical testing that could help bring more novel treatments to clinical trials.
Prognosis.
The prognosis for children with HGA is poor but is better than for adults. The degree of surgical resection is associated with better PFS. Patients with anaplastic astrocytoma have a more favorable prognosis than those with glioblastoma. A report from the CCG showed that the 5-year PFS rates for anaplastic astrocytoma were 44% ± 11% and 22% ± 6% for children who underwent GTR versus other types of surgery, respectively. The 5-year PFS rates for glioblastoma were 26% ± 9% and 4% ± 3% for children who underwent GTR versus other types of surgery, respectively. Patients younger than 3 years appear to have a survival advantage compared with older patients. One study showed worse PFS for patients initially treated with dexamethasone, raising the question of whether tumor inflammation may be important to treatment. Poor performance at diagnosis on the FMH health status scale correlates with a worse prognosis. The size of the treating medical center has no effect on prognosis. In terms of biologic markers, PTEN expression and MGMT methylation favorably affect survival, whereas p53, endoglin, and PARP positivity are negative prognostic markers. MIB1 staining is usually elevated in pediatric HGA and correlates with grade and prognosis. Patients with MIB1 levels greater than 36% had a 5-year PFS of 11% compared with 33% in persons with MIB1 levels less than 18%.
Special Cases.
HGAs occur more commonly in persons with certain genetic syndromes. These syndromes include hereditary nonpolyposis colorectal carcinoma and Li-Fraumeni syndrome, a dominantly inherited syndrome involving mutations of the p53 tumor suppressor protein. TP53 mutations are rare in sporadic pediatric CNS tumors lacking a typical family history. HGAs may also arise in persons with NF-1, although low-grade lesions are far more common; in one large case series, HGAs developed in 0.5% of patients.
Cases of de novo congenital HGAs have been observed at birth or within the first 3 months of life and show similarities in gene expression profiling compared with both pediatric and adult HGA. Prognosis, however, appears to be far better. Long-term survival, occasionally even without treatment, occurs in the majority of these patients and supports the unique characteristics of these otherwise fatal tumors.
Thalamic gliomas can occur as high-grade or low-grade lesions. Poor prognostic factors in these patients include a short interval of symptoms, poor early response to chemoradiotherapy, high-grade histology, and inability to resect the lesion. For spinal HGA the median age of presentation is 4 years. Positive prognostic factors include age younger than 5 years, anaplastic astrocytoma histologic features, lack of hemorrhage, and GTR. Multimodality treatment may have some effect. Another rare subtype of pediatric glioblastoma is giant cell glioblastoma. Although time to progression in this variant may be slightly longer, overall prognosis is not significantly better than in other pediatric HGAs.
Gliosarcomas are glioblastomas with a sarcomatous component that can affect children of any age but seem to be most frequent in infants and toddlers, as well as patients who have received prior radiation therapy. They most often present with increased ICP and are located in the cerebrum. Gliosarcoma also carries a poor prognosis. Optic nerve gliomas show HGA features on pathologic examination in approximately 17% of patients. School-aged children are most often affected. The 5-year OS rate is approximately 20% with high-grade histologic features, apparently irrespective of treatment with radiation or surgery.
Diffuse Intrinsic Pontine Gliomas
Tumors of the brainstem occur in approximately 10% of pediatric patients with CNS tumors. The majority of these tumors, approximately 80%, are classified as DIPG and are infiltrative, expansile lesions of the pons. Most of these tumors are anaplastic astrocytoma or glioblastoma on histopathologic examination, although grade II DIPG has been reported; the dismal prognosis is not affected by grade. This diagnosis must be distinguished from other pediatric brainstem tumors that are usually WHO grade I, well circumscribed as opposed to infiltrative, and carry a much improved prognosis. The median age at diagnosis of DIPG is 5 to 9 years, but these tumors may occur throughout childhood.
Lack of understanding of DIPG biology was a major roadblock in the past, mostly because researchers had minimal tissue to study because these tumors were rarely biopsied; the procedure was believed to be highly morbid. In addition many samples that were obtained were from areas at the periphery of the tumor and included normal cells and reactive gliosis. Even autopsy specimens were rare. However in more recent years, two major trends have emerged. First, researchers determined that obtaining DIPG tissue by autopsy was feasible, and provided an opportunity that families of these children generally greatly appreciated and did not regret. This tissue, generally preirradiated, led to new DIPG culture lines and biologic studies. Second, new neurosurgical techniques emerged, along with targeted therapies based on initial biologic studies, that could be offered to families who consented to upfront biopsy so that patients’ tumors could be tested for susceptible genetic changes.
With tissue available, studies of DIPG biology have emerged at a rapid pace. One theme across several studies is amplification of the region containing PDGFR alpha (PDGFRA), and protein expression analysis has confirmed the importance of PDGFRA deregulation in DIPG. Thirty-six percent of DIPG samples in another study had PDGFRA amplification, far more than in pediatric and adult HGA. Another key finding has been the discovery of novel mutations in H3F3A, encoding histone H3.3, or histone cluster 1, H3b (HIST1H3B) , encoding histone H3.1. These mutations were isolated by whole genome sequencing of DIPG samples. Of note, the H3F3A mutation was also found in 22% of nonbrainstem pediatric HGAs but was not found in adult HGAs. In a subsequent study in which investigators looked for these mutations in a targeted manner, it was found that the H3.3 mutation occurred in 71% of DIPGs. Although the biologic consequences of this mutation are not yet clear, it does seem to correlate negatively with prognosis.
Other new findings have included overexpression of Aurora kinase B, WEE1 G2 checkpoint kinase (WEE1), B7-H3 (CD276), and interleukin (IL)-13Rα2. Common mutations have been found in TP53, PI3K catalytic subunit (PI3KCA), and EGFR, which surprisingly showed expression of the vIII mutant in a majority of cases, although confirmation of this finding is needed. Authors of one study suggested two biologic subgroups of DIPG, one driven by proangiogenic changes and one driven by PDGFRA amplification or mutation; this latter group seemed to have more deaths before 10 months from diagnosis. Finally, with establishment of a plausible cell of origin that depends on the hedgehog pathway, as well as development of new preclinical models including mouse intracranial xenograft models, further understanding of the disease should be forthcoming.
Clinical Presentation.
The clinical presentations of children with brainstem tumors appear to fall into two groups. Patients with fewer than 3 months of symptoms, multiple cranial neuropathies (unilateral or bilateral), long tract signs (e.g., Babinski sign, hyperreflexia, and weakness), and cerebellar signs (e.g., ataxia, dysmetria, and dysarthria) are likely to have DIPG. Symptomatic hydrocephalus is present in fewer than 10% of patients with DIPG at presentation. Although the pons is not typically associated with behavior changes, pathologic laughter or separation anxiety have been symptoms noted in persons with DIPG. In contrast children with low-grade brainstem gliomas tend to manifest a more insidious history of isolated cranial nerve palsy or weakness, usually for longer than 3 months.
Imaging and Histology.
The diagnosis of DIPG may be made on the basis of the classic MRI appearance of a diffusely expanded pons with encirclement of the basilar artery. Most DIPGs appear to be hypointense on T1-weighted MRI and hyperintense on T2-weighted imaging. A prominent edema signal is common ( Fig. 57-32 ). The ventral pons may appear swollen and infiltrated. Contrast enhancement is variable, from homogeneous rim enhancement to patchy enhancement to complete absence of enhancement. In contrast to DIPGs low-grade focal lesions of the brainstem present with a more circumscribed appearance and associated contrast enhancement that occupies less than 50% of the axial diameter of the brainstem. These tumors may be composed of cystic and solid components. Leptomeningeal dissemination at diagnosis and recurrence may be more common in DIPGs than previously understood, and given the negative effect on prognosis, spine MRI at diagnosis should be considered. Diagnosis by imaging alone puts DIPG in a unique category, apart from nearly every other pediatric tumor, and some studies showing variability in reading of MRIs have called the practice into question. Another study of MRI-biopsy correlation showed that all diffuse, nonenhancing tumors were DIPG pathologically, whereas the diagnosis was more variable with focal or enhancing tumors.
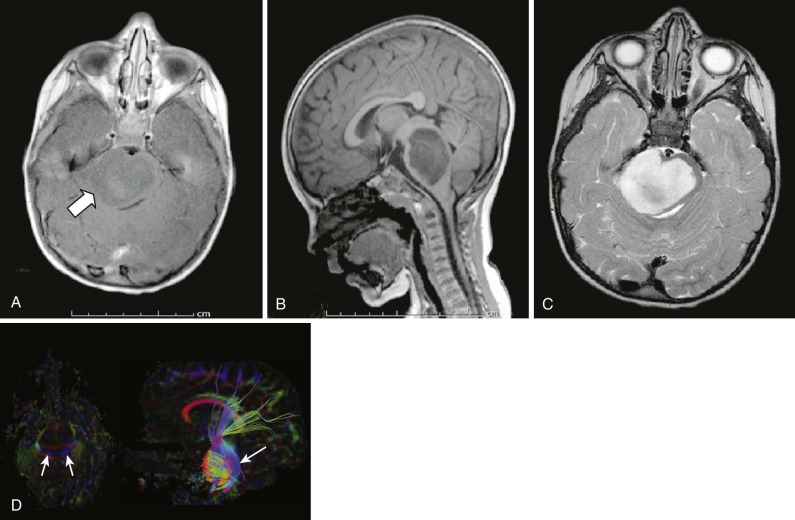
New MRI techniques have been found to have specific roles in diagnosing DIPGs. DTI is helpful in differentiating DIPG from demyelinating disease when the diagnosis is in question. Susceptibility-weighted imaging appears to be the best technique for detecting hemorrhages in DIPGs, which appear to be present in 47% of patients at diagnosis, although most hemorrhages are petechial and asymptomatic. MRS has identified a specific spectrum consistent with the malignant potential of DIPG. More importantly this method can detect positive responses in patients after radiation therapy, as well as changes indicative of progression in advance of MRI changes. The Cho : NAA ratio and lactate measurement on MRS appear to correlate with the length of survival. Elevated tumor perfusion on MRI after radiation therapy appears to predict a longer OS, and perfusion has been shown to decrease prior to clinical progression. Serial measurement and estimation of response may be best achieved with FLAIR imaging, although no radiologic methodology is optimal for disease assessment or response. Time to progression or OS, rather than radiographic response, may therefore be the best determination of treatment activity in clinical trials for this population. Ventriculomegaly without obstruction appears to be a common finding after radiation therapy in persons with DIPG, suggesting communicating hydrocephalus.
The histologic appearance of DIPG is similar to that of other HGAs. Most biopsy samples show evidence of atypia and mitoses and qualify as grade III anaplastic astrocytomas. Differentiation between anaplastic astrocytoma and glioblastoma in the pons is not relevant because all these patients appear to have an equally poor outcome.
Management.
During the past 30 years, little progress has been made in the treatment of DIPG. Radiation therapy has improved the median OS from weeks to months, but it can at best be considered palliative therapy. Despite many clinical trials, no chemotherapeutic agent has shown an improvement compared with radiation alone. Few patients survive more than 2 years.
Corticosteroids are generally started at the time of diagnosis, because peritumoral edema may contribute significantly to symptoms, and steroids may therefore offer some relief. Patients with DIPG often require steroids for a long duration, and investigation of agents that can offer relief from peritumoral edema without the adverse effects of steroids is ongoing.
The tide is clearly shifting toward diagnostic biopsy in patients with suspected DIPG. Several retrospective studies of open and stereotactic biopsy showed that the procedure is feasible and safe. A prospective trial of 24 patients undergoing stereotactic biopsy showed that transient cranial nerve palsies in two children were the only complications, with tissue obtained in all patients, including two for whom the biopsy results changed the management approach. A consensus statement in support of biopsy on behalf of a large group of DIPG experts has now been published. The procedure still carries risk, however, and should be performed at experienced centers by experienced neurosurgeons. The ideal locations for stereotactic biopsy appear to be areas of focal anaplasia, represented by T2 hypointensity, contrast enhancement, and diffusion restriction.
Radiation therapy remains the mainstay and standard treatment for DIPG. The tumor volume and a 1- to 2-cm volume of surrounding brainstem are generally included in the field, and conventional doses range from 5400 to 5940 cGy given over 6 weeks. This treatment is generally effective at decreasing tumor size, decreasing symptoms, and allowing patients to stop taking steroids, but these effects generally last only a matter of months, and the treatment is still considered palliative instead of curative. Multiple studies have attempted hyperfractionation techniques, ranging from 6600 to 7800 cGy, without improvement in survival ; this outcome may relate to the inherent resistance of these tumors. More recently in an attempt to reduce the impact of the radiation therapy schedule for these children with a limited life span, hypofractionation schedules have been tested, usually 3900 to 4500 cGy over 3 to 4 weeks, with a noninferior response rate and duration. Reirradiation is also the only therapy that has shown any potential efficacy at recurrence: two retrospective reports showed transient responses of some patients to reirradiation doses of 1800 to 2000 cGy, with children who had a prolonged duration of response from initial radiation therapy most likely to benefit.
Many adjuvant chemotherapy agents and strategies have been tried and have failed to improve outcomes in patients with DIPG compared with radiation therapy alone. Many recent phase II trials have centered on the use of TMZ, given that this agent is now standard of care in adults with HGG. However three phase II trials of TMZ given with radiation therapy, followed by TMZ alone, have failed to show an increase in survival time compared with historic standards from radiation therapy alone, and the addition of TMZ worsened toxicity. Other recent phase II trials have shown no benefit of tamoxifen, pegylated interferon α-2b, a regimen of cisplatin, etoposide, ifosfamide, vincristine, and valproate, and inhaled carbogen as a radiosensitizer. Other past failed agents and strategies have included preirradiation multiagent chemotherapy, topotecan as a radiosensitizer, etanidazole as a radiosensitizer, chemotherapy concurrent with radiation therapy including thalidomide, trofosfamide and etoposide, carboplatin, carboplatin and etoposide, TMZ and cis-retinoic acid, chemotherapy with BBB disruption, intraarterial bevacizumab injection, and a postradiation high-dose chemotherapy regimen. New strategies that have been the subject of recent phase I reports include the use of the PDGFR inhibitor dasatinib in combination with the VEGFR2 inhibitor vandetanib, vandetanib alone, and the use of arsenic trioxide. The development of nonhuman primate models of direct drug delivery into the brainstem opens another avenue for developing improved therapies for these tumors.
Prognosis.
DIPG remains the most challenging of pediatric tumors to cure. Despite all approaches most survival curves are superimposable with regard to OS. Meta-analyses of numerous clinical reports indicate that median PFS ranges from 5 to 9 months, with a median OS of 6 to 16 months. One-year and 2-year survival ranges from 17% to 50% and 7% to 29%, respectively, with less than 10% survival at 3 years. Neither MRI characteristics nor WHO grade has an impact on prognosis in persons with DIPG. Poor performance at diagnosis on the FMH health status scale correlates with a worse prognosis. The size of the treating medical center has no effect on prognosis. One report indicated an improved prognosis for patients with DIPG who are younger than 3 years, although questions of misclassification of patients with low-grade brainstem glioma as having DIPG inevitably arise with reports of improved survival—yet another reason for the use of diagnostic biopsy. Very few cases of neonatal DIPG have been reported, and outcomes in these reports have been inconsistent, ranging from spontaneous regression to death within weeks. Retrospective studies have shown worse prognoses for patients initially treated with dexamethasone or rofecoxib, raising the question of whether tumor inflammation may be important to treatment. Patients with leptomeningeal dissemination at diagnosis appear to have a shorter PFS than do patients who do not have this finding. It is hoped that with time, the greatly improved understanding of DIPG biology and promising preclinical models will soon lead to successful clinical trials and an improved outlook for patients afflicted with this terrible disease.
Gliomatosis Cerebri
Although much more common in adults, gliomatosis cerebri has also been identified in children. Similar to adults, in children these lesions are often grade III astrocytomas that diffusely infiltrate the brain and appear to favor white matter tracts, and they can easily cross the corpus callosum. In spite of the widespread presence of disease, often involving both hemispheres and multiple lobes, a large primary focal mass is usually absent. All parts of the brain and spine can be involved. The overall prognosis is very poor by virtue of the disease’s diffuse nature and unresectability. Patients can be kept alive with palliative care for longer than would be expected for widely diffuse lesions, suggesting a different biology from that of typical grade III astrocytomas. Genetic aberrations, including loss of chromosomes 13q and 10q or gains of chromosome 7q, have been shown to be markers of poor outcome. Some tumors demonstrate abnormalities in the p53 pathway. Recently IDH1 mutations have also been found in adult but not pediatric gliomatosis cerebri.
Clinical Presentation.
The clinical presentation of children with gliomatosis cerebri is varied and often subtle, mimicking a number of neurologic conditions. Children present more frequently than adults with refractory seizures that can be palliated with surgical intervention. Other presentations of this disease include raised ICP and cognitive impairment. Tumors involving the brainstem can result in cranial nerve deficits, and those in the optic pathway can present with visual field deficits.
Imaging and Histology.
Gliomatosis cerebri is diagnosed on MRI scan and demonstrates a characteristic diffuse infiltrative pattern. T1-weighted sequences are usually isointense to hypointense and underestimate the extent of disease, whereas T2-weighted sequences are hyperintense and highlight the full extent of the disease ( Fig. 57-33 ). Signal abnormality usually involves multiple lobes. The presence of contrast enhancement is strongly correlated with a poorer prognosis in both adult and pediatric patients. A solid area of tumor is usually not evident. MRS demonstrates elevation of choline and a decrease in NAA levels. Although the hallmark of gliomatosis cerebri is an infiltrative astrocytic tumor, areas of oligodendroglial cells are not uncommon for adult and pediatric patients. Most tumors are consistent with grade III histology, and mitoses are often present. MIB1 staining can vary considerably. The absence of mitoses is more likely to be because of sampling error. GFAP and S-100 can be positive or negative. Nestin and vimentin can be positive. Because of their infiltrative growth and co-opting of existing blood vessels, these tumors lack the abundant neovascularization observed in other malignant gliomas, which suggests that they will not be effectively treated with antiangiogenic agents.
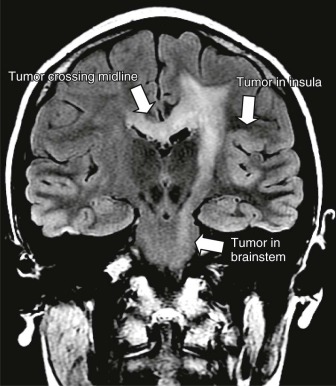
Management.
Currently patients with gliomatosis undergo biopsy to confirm the diagnosis. Given the diffuse nature of these tumors, complete resection is rarely feasible. The mainstay of therapy is irradiation to the involved region, which, for pediatric patients, may require almost complete whole-brain therapy. In addition to significant morbidity, large-volume radiation therapy for gliomatosis cerebri has not been proven to be effective, although it may delay the time to progression. Objective responses to TMZ have been documented in adults and, in oligodendrogliomas, correlate with chromosome 1p and/or 19q loss. The impact of MGMT expression on response and outcome has not yet been determined.
Prognosis.
Pediatric patients with gliomatosis cerebri have a poor outcome. In a large series of pediatric and adult patients, a median survival of 14.5 months was demonstrated. Younger age, lower grade histology, and chemoresponsiveness were associated with slightly longer survival times. Although long-term survival is unlikely, adults whose tumors have chromosomal 1p and/or 19q loss can show more chemoresponsiveness to agents such as TMZ. Similar activity in pediatric patients has not been reported because of the rarity of these tumors in this population.
Ependymomas
Ependymomas are glial tumors that arise from ependymal cells that line the ventricles in the CNS. Ependymal cells play an essential role in the transport of CSF. Experimental evidence has indicated that these cells may be derived from radial glia. Ependymal tumors represent approximately 10% of all childhood intracranial neoplasms, constituting the third most common pediatric brain tumor, after astrocytomas and medulloblastoma. They are equally distributed between males and females, and the median age at diagnosis is approximately 6 years. They are significantly less common in blacks than whites.
Ninety percent of pediatric ependymomas are intracranial, with 66% to 75% arising from the posterior fossa. Supratentorial ependymomas are often located in the brain parenchyma, away from the ependymal surface, in contrast to infratentorial ependymomas, which are usually located in relation to the fourth ventricle. Spinal cord ependymomas represent fewer than 10% of pediatric intramedullary spinal tumors. In contrast ependymomas represent more than 50% of intramedullary spinal tumors in adults.
The WHO classification system recognizes four major types: subependymoma and myxopapillary ependymoma (WHO grade I), classic ependymoma (WHO grade II), and anaplastic ependymoma (WHO grade III). Differentiation of grade II and grade III ependymoma does not clearly have an influence on outcome, in contrast to the grade in astrocytic tumors. Subependymomas are well circumscribed, slow-growing, and often asymptomatic intraventricular neoplasms that are usually found incidentally in middle-aged and older adults or at autopsy. Myxopapillary ependymomas (WHO grade I) are also slow-growing tumors of the conus medullaris, cauda equina, and filum terminale and manifest primarily in young adults. They account for approximately 10% of all ependymomas and portend an overall good prognosis with surgery-only approaches.
Recent advances in molecular studies have found that ependymomas from different compartments of the brain and spine have unique molecular signatures with distinct patterns of gene expression, chromosomal changes, and protein expression. Deletion of tumor suppressor P16INK4A (located at chromosome 9p21.3) was frequently observed in supratentorial ependymoma. Witt et al analyzed the transcriptome of 177 patients with posterior fossa ependymoma and proposed two distinct subtypes of posterior fossa ependymoma based on different clinical and molecular characteristics (i.e., age, tumor location, degree of genomic instability, alterations in molecular pathways, and prognosis). The “pediatric” subgroup (median age of 2.5 years), designated as group A in the study, showed relatively less genomic instability but commonly harbored chromosome 1q gain and 22q loss. A balanced genomic profile and chromosome 1q amplification have been previously described as two of three defining molecular features of posterior fossa ependymoma. The older subgroup (median age of 20 years), designated as group B, exhibited a much higher degree of genomic instability and extensive cytogenetic aberrations. Interestingly a balanced genomic profile, seen in the pediatric subgroup, was paradoxically associated with a worse prognosis. Posterior fossa ependymomas in young children were much more likely to recur, metastasize, or result in mortality. Even accounting for whether GTR was achieved, group A tumors exhibited worse PFS and OS compared with group B (18% 5-year PFS and 52% OS in group A vs. 91% 5-year PFS and 100% OS in group B; P < .0001). The identification of immune markers in these analyses may provide important opportunities for the development of immunotherapy-based treatments. Chromosome 22q loss is commonly associated with spinal ependymoma in adults and children.
The most consistent genetic defects in ependymoma are monosomy 22 or structural abnormalities of chromosome 22q, which raises the possibility that a tumor suppressor gene may be located on chromosome 22. As previously noted, comparative genomic hybridization has demonstrated significant differences between infant and childhood ependymoma, suggesting that the pathogenesis of this disease differs by age and location. A number of other molecular defects have been described in ependymomas, including the abnormal expression of ERBB2 and ERBB4 receptors, defects in the p53 homologue p73, increased expression of the VEGF protein, Nestin expression, PI3K activation, immune targets such as EphA2, IL-13Rα2, and Survivin, amplification or overexpression of the p53 regulator MDM2, overexpression of cyclooxygenase-2 (COX-2), the presence of telomerase activity, and expression of protein 4.1. Other molecules implicated in the pathogenesis of ependymoma have been reported by using micro RNAs and proteomic fractionation of tumor tissue. Future studies will be required to determine the significance of these molecules and their potential use as diagnostic markers and targets for therapy.
Significant progress has also been made in the use of these markers in attempting to understand and characterize the putative ependymoma stem cell with expression of OLIG2. The role of telomerase may also be an important factor in the recurrence of ependymomas, and additional markers are being developed. The advances in assessment of the epigenome of ependymoma have also recently been reported and give important clues about the role of DNA methylation in the genesis and possibly targets of ependymoma. Finally, the presence of JC (John Cunningham) viral sequences identified in 5 of 18 ependymomas (but 0 of 32 medulloblastomas) raises the possibility that this agent is associated with ependymal tumorigenesis. Simian virus 40 (SV40) viral, large, T-antigen sequences have also been identified in ependymomas and choroid plexus papillomas (CPPs) but are negative in adjacent normal brain. Although these results have generated significant discussion, they have not been confirmed. Further studies will be needed to validate these findings.
In myxopapillary ependymoma, a WHO grade I ependymoma that is confined to the conus medullaris-cauda equina-filum terminale of spinal cord, upregulation of 20 members of the homeobox (HOX) family that are not typically observed in intracranial ependymoma has been described. EGFR has also been identified in ependymomas of the brain and myxopapillary ependymoma and correlates with progression.
Persons with NF-2 have an increased susceptibility to intramedullary spinal cord ependymomas. Although the NF2 gene is located at chromosome 22q12, mutations in NF2 (Merlin) are rarely found in sporadic ependymomas. A recent microarray analysis of pediatric ependymomas has identified a cluster of genes distinct from NF2 that may be involved in ependymoma tumorigenesis. Expression profiling has indicated that histologically similar ependymomas from different parts of the CNS are molecularly and clinically distinct disease subgroups. Certain familial colon cancer syndromes may also be associated with an increased incidence of ependymoma in offspring.
Clinical Presentation.
The presenting symptoms of infratentorial ependymomas are a result of their origin from ependymal tissue lining the fourth ventricle. Hydrocephalus results when the tumor fills the fourth ventricle, causing obstruction of the normal spinal fluid flow and subsequent development of headache, irritability, nausea, vomiting, and ataxia. Papilledema can often be found on physical examination. Tumors that extend out of the foramen of Luschka may compromise lower cranial nerve function and cause hearing impairment, hoarseness, and/or dysphagia. If the tumor extends through the foramen of Magendie, the patient may report neck discomfort or be noted to have torticollis. The most common signs of the tumor in infants are irritability, decreased oral intake, vomiting, ataxia, head pain, lethargy, and increased head circumference. Supratentorial ependymomas which represent approximately one third of ependymomas, are more common in older children and adults. These patients present with focal neurologic deficits and seizures. Spinal ependymomas are typically located in the cervical region. The most common presenting symptom is pain or motor deficits localized to the level of the spinal cord lesion. The pain is typically described as being worse at night, presumably because of congestion of the spinal venous plexus that occurs when the patient is in the recumbent position. The second most common symptom is radicular dysesthesias, and a late manifestation of this symptom is progressive spastic quadriparesis. Thoracic ependymomas are associated with scoliosis. Myxopapillary ependymomas of the conus medullaris and filum terminale may cause low back pain, radicular pain, saddle anesthesia, and sphincter dysfunction. When these tumors disseminate, they usually remain in the spine, although cranial metastases have been reported; therefore craniospinal imaging at diagnosis should be undertaken. Pediatric myxopapillary ependymoma may have a higher propensity to spread compared with adult tumors.
Imaging and Histology.
A typical MRI appearance of a fourth ventricular ependymoma is that of a homogeneously enhancing well-circumscribed solid mass, extending out one of the foramina of Luschka or Magendie with obstructive hydrocephalus. These tumors classically wrap around the brainstem or herniate through the foramen magnum ( Fig. 57-34 ). Hemorrhage and calcifications can be observed and are more common in supratentorial lesions. Ependymomas may occasionally spread via CSF, seeding the leptomeninges or ventricles, either at diagnosis or at recurrence. Careful attention to staging of patients is therefore important.
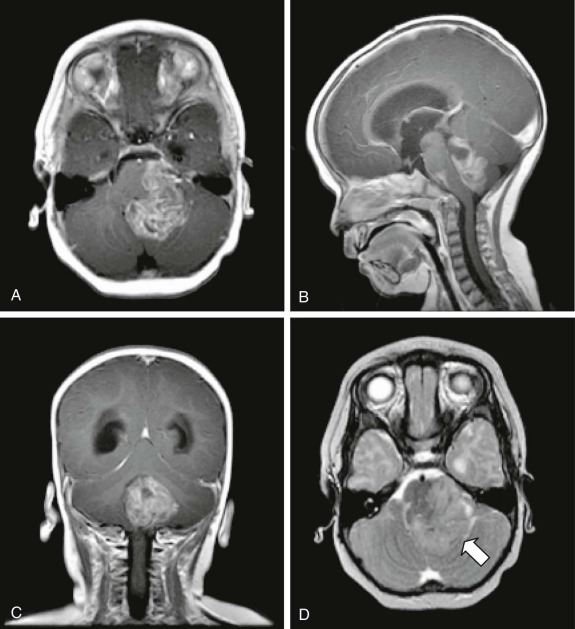
The characteristic microscopic feature of a classic ependymoma is that of dense cellularity, intermixed with perivascular pseudorosettes consisting of tumor cells and surrounding a neoplastic blood vessel ( Fig. 57-35 ). True ependymal rosettes representing abortive canals are relatively uncommon. Histologically the anaplastic variant is recognized by the presence of mitoses, necrosis, and vascular proliferation. They tend to be more cellular than grade II ependymomas but usually remain well demarcated. MIB1 rates greater than 5% in incompletely resected tumors or greater than 15% in completely resected tumors correlate with more aggressive behavior. GFAP and S-100 are positive in most ependymomas, and epithelial membrane antigen (EMA) stains are positive in anaplastic variants. The presence of perivascular elastic fibers may also be a diagnostic feature of these tumors. In patients in whom the diagnosis of ependymoma is indeterminate, electron microscopic analysis of cilia structure and junctional complexes, which are found in most ependymomas, can be used to confirm or exclude the diagnosis. Myxopapillary ependymomas have a mucinous appearance and arise almost exclusively in the cauda equine. Their MIB1 labeling index is typically low. Tanycytic ependymomas are a rare subtype of ependymoma that can occur throughout the brain or spine. Other rare subtypes include cellular ependymoma, papillary ependymoma, and clear cell ependymoma variants.
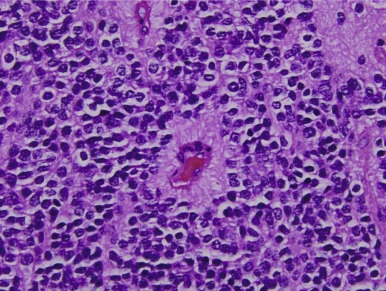
Management.
Evaluation of patients at diagnosis or recurrence should include an MRI of the brain and spinal cord, as well as cytologic evaluation of the CSF, usually performed at least 14 days after the operation. Attempting CSF analysis during the surgery or shortly after could give false-positive results. The usefulness of CSF sampling in patients may not be particularly sensitive. A number of factors have been associated with an unfavorable outcome, including younger age at diagnosis, subtotal resection, and a high MIB1 labeling index. Anaplastic histology remains controversial as a negative prognostic factor. Of these factors the single most important factor in the determination of prognosis appears to be whether a complete resection can be accomplished.
The first line of treatment is surgery with the goal of GTR. If a complete resection with clear margins of a grade II spinal cord or supratentorial ependymoma can be achieved, adjuvant therapy can be deferred in some patients. Technologic advances such as the operating microscope, Cavitron ultrasonic aspirator, intraoperative ultrasound, and intraoperative MRI, as well as electrophysiologic monitoring, have facilitated the safety of resections of intraaxial spinal cord tumors while reducing operative morbidity. Overall, spinal cord ependymomas are more easily resectable than astrocytomas because of the presence of a better demarcated cleavage plane. Tumors with an infiltrative boundary, or those with anaplastic histology, require adjuvant therapy. All posterior fossa tumors, independent of grade and degree of resection, should also receive adjuvant treatment. Spinal myxopapillary ependymomas with a complete surgical resection have an excellent prognosis and do not usually require adjuvant therapy even in the presence of drop metastases in the thecal sac. Given the overall good prognosis of these tumors, the need for radiation in incompletely resected disease is unclear, and careful surveillance imaging is needed. Early radiation therapy is not always routinely used but is recommended by some persons.
Regarding radiation therapy, there is a long history of deferring radiation therapy for infants and young children who have posterior fossa ependymomas, with use of various conventional chemotherapy regimens instead. Previous treatment recommendations included radiation therapy for patients in whom gross resection was not achieved or for patients with recurrent disease. However partially resected tumors almost invariably recur, requiring further surgery, radiotherapy, and conventional chemotherapy. As such, in the United States the current strategy for ependymoma is to recommend deferral of adjuvant therapy in persons with completely resected supratentorial ependymoma, use of radiotherapy in the involved fields alone for completely resected posterior fossa ependymoma, and use of multiagent chemotherapy for subtotally resected ependymoma, followed by second-look surgery and involved field radiation therapy. The benefit of hyperfractionation has not been established. Wide-field pulsed reduced dose rate radiotherapy is currently being evaluated. Craniospinal radiation therapy does not appear to be superior to focal radiation therapy. Several studies have suggested that radiotherapy prolongs PFS after subtotal resection of an ependymoma. Deferral of radiation therapy after initial complete resection can result in reduced cure, even when complete resection is achieved a second time. Proton radiation therapy appears to have equal efficacy when compared with photon therapy; it can reduce the potential long-term toxicity by decreasing the volume of normal brain tissue that is also targeted and is therefore recommended over photon therapy if it is available locally.
Until recently there was no clear role for chemotherapy in the management of ependymomas outside of clinical trials. Several small series in newly diagnosed and recurrent disease have shown objective responses to the following drugs: carboplatin, cisplatin, ifosfamide, and etoposide. Chemotherapy is more often used for infants and younger children with incompletely resected or disseminated disease. Encouraging results have been reported from the most recent cooperative group clinical trial involving preirradiation chemotherapy, which showed a 40% complete response (CR) rate in patients who received preirradiation chemotherapy for residual postoperative tumor. The two factors associated with a favorable outcome were the achievement of a complete resection and supratentorial disease. Chemotherapy should therefore be used to achieve a CR or improve the chances of a complete surgical resection at second-look surgery. It is not highly effective as sole treatment for this disease, and recurrences occur relatively rapidly in a significant percentage of patients if radiotherapy is not administered early. Infants may be more responsive, and 23% to 40% can be cured with chemotherapy alone. The use of high-dose chemotherapy and stem cell rescue in infants with ependymoma has failed to demonstrate a significant advantage compared with standard modalities in most, although not all, studies. Although uncommon, up to 10% of patients can have leptomeningeal spread of ependymoma. Response to intrathecal liposomal cytarabine has been reported.
Recurrences of intracranial ependymoma can occur throughout the first decade after initial therapy, although most recur within the first few years. Surveillance scanning is important because it allows smaller asymptomatic tumors to be identified that may be more amenable to reresection. Most recurrences are within the original radiation field and do not appear to be related to marginal failures.
Prognosis.
The most important prognostic factors for intracranial and spinal cord ependymoma are age, tumor grade, extent of surgical resection, and delivery of radiation therapy with doses of at least 5400 cGy. Children younger than 3 years, those with WHO grade III disease, or those with less than a GTR have been shown to have lower survival rates. The 5-year PFS and OS for patients with subtotal versus total resection of posterior fossa ependymomas is 25% and 66%, respectively. The prognosis of patients with disseminated disease is worse. Patients with a supratentorial nonanaplastic ependymoma can be treated with surgery only if the tumor can be completely resected. Recently a molecular assay for HTERT expression has been shown to predict the likelihood of progression and survival of pediatric intracranial ependymoma. Additional studies on the molecular profile of ependymomas will likely lead to improved stratification and prognostication of patients as previously discussed. Patients with an elevated MIB1 labeled index of more than 5% in incompletely resected ependymomas or of more than 15% in completely resected ependymomas were reported to have a worse prognosis in one study. Patients with a deletion of 6q15.3 appear to have an improved outcome. Molecular profiling has identified that the group A ependymomas (posterior fossa, infants) have a more aggressive biologic behavior and worse prognosis when compared with the group B ependymomas (supratentorial), although this classification is not currently being used to guide therapy intensity at the present time.
Most patients with completely resected ependymoma followed by focal radiation therapy will be long-term survivors. Consideration of advanced radiation planning and the type of radiation delivered (i.e., proton vs. photon) are critical to minimize long-term neurocognitive morbidity in this population.
At the time of recurrence, no standard salvage regimen has been proven to be effective. Reoperation to achieve a complete resection has been curative in some patients and is typically the first modality considered ; this approach can be combined with reirradiation. SRS has also been used with some success for focal recurrences of ependymoma. Patients with metastatic disease or infiltration into the brainstem or other critical structures have a poor salvage rate, presumably because of the lack of good therapies for recurrent disease and the inoperable nature of the lesions. Oral etoposide alone or in combination as a metronomic therapy combination has demonstrated responses in this disease. Bevacizumab in combination with irinotecan did not show any significant activity. A single case report of a response to the mTOR inhibitor sirolimus has also been published.
Recurrence of myxopapillary ependymoma can occur after complete resection and can be associated with dissemination. Although cranial metastases have been reported, most cases result in regional metastases of the spine but not beyond the foramen magnum. Many of these patients can undergo salvage therapy with radiation. Consequently frequent surveillance scanning of patients with myxopapillary ependymoma is recommended to identify recurrent disease early.
Oligodendrogliomas
Oligodendrogliomas are rare brain tumors in children, representing fewer than 1% of all pediatric brain tumors. The mean age at diagnosis is approximately 10 to 13 years, with a male predominance. The WHO grading system recognizes two grades for oligodendroglial tumors: well-differentiated grade II oligodendroglioma and grade III anaplastic oligodendroglioma. As with other glial tumors, higher grade is a predictor of decreased OS. Other reported markers in oligodendrogliomas include deletions of the cyclin-dependent kinase inhibitor 2A ( CDKN2A ) gene on chromosome 9p, mutations in TP53 and PTEN , and amplification of the EGFR. However it is not known whether these factors are predictive of outcome in pediatric patients. Unlike adult tumors, pediatric oligodendrogliomas do not typically have the chromosome 1p or 19q deletions or mutations in TP53 or IDH1 .
Clinical Presentation.
Oligodendrogliomas are diffusely infiltrating tumors that tend to occur in the white matter of the cerebral hemispheres. Patients frequently present with seizures, although higher-grade tumors may present with evidence of increased ICP because of more rapid growth. Other symptoms at presentation can include headache, visual field defect, paresis, and cranial nerve palsy. Although uncommon, oligodendrogliomas can also occur throughout other areas of the brain, including the posterior fossa, brainstem, and spine.
Imaging and Histology.
MRI evaluation of oligodendrogliomas typically reveals increased T2-weighted signal and decreased T1-weighted signal. Gadolinium contrast enhancement is more common in tumors that grow as solid masses and less common in purely infiltrative tumors. Contrast enhancement is also more common in grade III than in grade II oligodendrogliomas. Although oligodendrogliomas bear morphologic similarity to oligodendrocytes, the cellular origin of these tumors has remained difficult to prove. Histologic examination of fixed specimens of oligodendrogliomas reveals a monotonous pattern of cells with round nuclei and clear perinuclear halos (a “fried egg” appearance). This appearance is an artifact of formalin fixation and is not seen in frozen sections or tumor smears. Higher-grade anaplastic oligodendrogliomas (WHO grade III) are characterized by increased nuclear variability, increased mitotic activity, and/or microvascular proliferation. These features can occur diffusely throughout the tumor or in discrete foci. The differentiation between oligodendrogliomas and other diffuse gliomas such as astrocytomas and OAs is difficult because of a lack of reliable molecular markers that distinguish oligodendroglial tumors from astrocytic tumors. Mature oligodendrocyte-specific markers such as myelin basic protein, 2′3′−cyclic-nucleotide 3′-phosphodiesterase, and myelin-associated glycoprotein are not expressed in oligodendrogliomas, although astrocytic markers such as GFAP and S-100β are expressed in both astrocytomas and oligodendrogliomas. More recently the lineage markers OLIG1 and OLIG2 have been evaluated in the hope that they may enable specific identification of oligodendroglial tumors. However despite the restriction of OLIG2 expression to normal oligodendrocytes and their precursors, OLIG2 stains all diffuse gliomas and precludes specific immunohistochemical classification of these tumors. The loss of chromosome 1p and/or 19q is commonly seen in adult oligodendrogliomas and correlates strongly with chemotherapy responsiveness and outcome. Pediatric oligodendrogliomas have a much lower incidence of chromosomes 1p and/or 19q deletions. Even in children in whom these deletions are identified, the greater chemoresponsiveness observed in adults is less evident.
Management.
Treatment of pediatric oligodendrogliomas, like other glial tumors, involves surgical resection, radiation therapy, and chemotherapy. The goal of surgery is to facilitate accurate diagnosis and to remove as much of the tumor as safely possible. In the adult literature, there is uncertainty as to whether the extent of resection correlates with OS. Although some larger case series have shown no prognostic value in the extent of resection, most evidence seems to indicate that the extent of resection may be associated with better prognosis. In pediatric patients, it appears that the extent of resection is a sensitive predictor of outcome. Fewer pediatric low-grade oligodendroglial tumors progress to high-grade tumors, so it is possible that patients with subtotally resected tumors may still have a good outcome. For example in one series of 20 patients with oligodendrogliomas treated with surgery only, 70% remained progression-free at a median of 5 years.
Despite the fact that oligodendroglial tumors are radiosensitive, the use of adjuvant radiation therapy in asymptomatic children with incompletely resected oligodendrogliomas is not favored because of the well-described late effects of radiation therapy. Children who are symptomatic, who have tumors in critical locations such as the brainstem, or who have progressive disease despite resection and/or chemotherapy may benefit from radiotherapy. However it is now clear that a subset of these children can be effectively treated with chemotherapy, allowing for a delay in, or avoidance of, radiation therapy. The combination of carboplatin and vincristine chemotherapy has demonstrated activity in various low-grade astrocytomas, regardless of histologic subtype. This therapy should therefore be considered for patients with oligodendroglioma. Older children may also benefit from chemotherapy. Based on overlapping activity in children with low-grade astrocytomas, the combination of TPCV might also be of use in patients who do not respond to or have disease progression after vincristine and carboplatin therapy. The combination of procarbazine, lomustine, and vincristine or TMZ monotherapy could also be considered based on the responses seen in adults, but these treatments have not yet been adequately tested in children.
In patients with anaplastic oligodendroglioma or progressive disease, despite surgery and adjuvant chemotherapy, the risks of radiation therapy may be offset by the risk of further tumor growth. A recent survey of adult neurooncologists has found that the most commonly recommended treatment for anaplastic oligodendroglioma is the use of concurrent TMZ and radiotherapy followed by adjuvant TMZ. In rare cases of dissemination, the overall prognosis for these patients is poor, although transient chemotherapy responsiveness can be achieved. Patients with oligodendroglial gliomatosis cerebri have a very poor prognosis.
Prognosis.
Overall long-term survival from low-grade oligodendrogliomas appears comparable with that of other LGGs. It should be noted, however, that only limited numbers of case series are available, making comparisons among studies difficult. Bowers and colleagues have reported an OS of at least 5.5 years in a series of 20 patients with low-grade oligodendrogliomas treated initially with surgical resection alone. Six of 20 patients in this series had disease progression at a median of 2.2 years after initial resection. Other studies have reported 5-year OS rates of 65% to 84.4%, although the proportion of patients treated with radiation therapy and/or chemotherapy differed among these studies. Children younger than 3 years and children with incompletely resected tumors may do worse. The rarity of anaplastic oligodendroglioma in children makes specific estimates of survival in pediatric patients difficult. Oligodendrogliomas that are more centrally located do not appear to do as well as those located more peripherally in the cortex.
A number of studies from adult patients have indicated that allelic losses at chromosomes 1p and 19q, typically involving the entire chromosomal arm at both sites, correlate with histologic classification, response to treatment, and prognosis. Loss at chromosome 1p is a predictor of chemosensitivity, and loss at combined chromosomes 1p and 19q is associated with both chemosensitivity and longer recurrence-free survival. Recent evidence has indicated that loss at chromosomes 1p and 19q is associated with MGMT promoter methylation and lower expression of MGMT. Interestingly these chromosomal features appear to be less common in pediatric oligodendrogliomas. One study has reported the lack of allelic loss in children younger than 9 years, although a modest number of tumors from children older than 9 years had losses of chromosome 1p (45%) and/or 19q (27%). Nevertheless the incidence of chromosomal losses is significantly less than that seen in adults (50% to 90%). Taken together these studies suggest that the oncogenesis of these tumors is distinct from their adult counterparts.
Oligoastrocytomas
OAs are tumors containing a mixture of two distinct neoplastic cell types that morphologically resemble the tumor cells of oligodendrogliomas and diffuse astrocytomas. These tumors correspond histologically to WHO grade II. The demography of OAs and anaplastic OAs (WHO grade III) is difficult to ascertain because of a high variability in the histopathologic criteria used for classification of these tumors. Clinically these tumors present with signs and symptoms similar to those of astrocytomas and oligodendrogliomas. Tumors arise most frequently in the cerebral hemispheres and lack any neuroradiologic features that would facilitate distinguishing them from oligodendrocytomas. Histologic diagnosis requires the recognition of the two different glial components, both of which must be neoplastic. Anaplastic OAs are WHO grade III oligoastrocytomas with histologic features of malignancy, including high cellularity, high mitotic activity, and increased nuclear atypia and pleomorphism. Currently treatment for OAs and anaplastic OAs is similar to that for other grade II and III glial lesions—surgical resection with adjuvant chemotherapy and/or radiation therapy for higher grade or progressive lesions.
Embryonal Tumors
Embryonal tumors represent a large and important fraction of pediatric brain tumors, both for their clinical impact and the importance of the scientific insights gained through their study. The most common embryonal tumors include medulloblastoma, pineoblastomas, and CNS PNETs. A number of other tumors are grouped into this category, such as ATRTs, ependymoblastomas, and medulloepitheliomas (see Table 57-6 ). Recent advances in gene expression array technologies have facilitated a new understanding of the molecular features of tumors within this set of diseases. The grouping of these tumors into the larger category of CNS PNETs will continue to evolve while a better understanding of their origin progresses. Currently all these tumors share a common property: high risk of dissemination and therefore the need to treat the entire craniospinal axis. It is expected that new technologies will soon allow for a better understanding of the cell of origin of the different tumor types. These considerations are no longer intellectual exercises. Gene-based classification systems may be the key to improved stratification and management and will likely augment, if not replace, the current histopathology-based classification system that underlies current treatment protocols.
| Tumor Location | Histologic Classification | Histologic Subtype |
|---|---|---|
| Fourth ventricle | Medulloblastoma |
|
| Cerebellar neuroblastoma * | ||
| Atypical teratoid-rhabdoid tumor | ||
| Pineal | Pineocytoma | |
| Pineoblastoma | ||
| Atypical teratoid-rhabdoid tumor | ||
| Supratentorial or intratentorial | Primitive neuroectodermal tumor * | |
| Cerebral neuroblastoma | ||
| Atypical teratoid-rhabdoid tumor | ||
| Ependymoblastoma † |
* The nomenclature for neuroblastoma and primitive neuroectodermal tumor (PNET) in the central nervous system results from the historic grouping of these tumors with other small round blue cell tumors of the body. Primary cerebral or cerebellar neuroblastoma are unrelated to similarly named tumors in the body and do not possess the abnormalities in the N-Myc pathway. Similarly central nervous system PNET is unrelated to Ewing sarcoma PNET and does not possess the classic chromosome 11;22 translocation.
† Ependymoblastomas were previously grouped with ependymomas but are now differentiated from this group as a result of their embryologic development and patterns of spread.
The history of small round blue cell tumors is a fascinating story that spans the evolving approach to cancer during the past century. Histopathology was, and remains, the cornerstone of all classification schemas. Tumors from different sites that appeared the same under microscopic examination were assumed to be similar tumors arising from different organs. With the introduction of immunohistochemical techniques of pathologic specimens, pathologists began to define important differences among populations. Small round blue cell tumors within the body were now divided into unique categories based on the presumed cell of origin. Tumors such as lymphoma, neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, and others became easily differentiated. The classification of small round blue cell tumors of the brain, by contrast, took a different direction. Medulloblastoma, a small round blue cell tumor of the cerebellum, was previously differentiated from other similarly appearing tumors based on its location within the posterior fossa, but some investigators thought that this arbitrary separation was unwarranted. Hart and Earle attempted to classify all embryonal tumors based on the expanding array of immunohistochemical markers and variations in the patterns of staining among these tumors. Rather than clarifying the classification of embryonal tumors, further blurring of the understanding of these tumors resulted. One contributing factor involved differing definitions for the grouping of embryonal tumors. For some investigators, medulloblastoma was considered a PNET and thus was lumped with pineoblastomas and sPNETs, whereas for other investigators, medulloblastoma remained a separate category. Another factor was the considerable variability in the quality of the staining pattern and heterogeneity within different areas of a single tumor and within different tumors of the same type. With advances in molecular pathology, classification has moved away from the simple microscopic appearance of a tumor to one that incorporates the pathways active in the tumor. Molecular signatures for these different tumors have been demonstrated; these signatures have shown that the tumors arise from different cell populations and, as a result, these tumors will likely require different treatments. Many prior treatment protocols grouped all these heterogeneous tumors together, which has confounded the true efficacy of the therapy under consideration and the prognoses for this heterogeneous group of tumors.
Medulloblastomas
Medulloblastomas represent approximately 15% of all pediatric brain tumors and approximately one third of posterior fossa tumors. In addition medulloblastoma accounts for more than 50% of pediatric embryonal intracranial tumors. The incidence in males is twice that of females, and the median age at diagnosis is 5 to 7 years, with most cases diagnosed in the first decade of life. The current standard of care for children diagnosed with medulloblastoma involves maximal surgical resection and adjuvant therapy with radiotherapy and chemotherapy. Radiation therapy involves a boost to the tumor site with craniospinal prophylaxis. Chemotherapy is administered both concurrently with radiation therapy and as multiagent maintenance chemotherapy after the completion of radiation therapy. Approximately 80% of children with medulloblastoma are cured, but current treatment strategies result in significant long-term morbidity.
With recent advances in transcriptome and genomic profiling of medulloblastoma, it has become apparent that medulloblastoma encompasses a heterogenous group of tumors with distinct pathologic features and clinical outcomes. These findings represent exciting prospects for the development of novel tumor-directed therapies and treatment strategies that are stratified according to the genetic profiles of the individual medulloblastoma.
Different subtypes of medulloblastoma are thought to originate from distinct germinal zones within the cerebellum—the EGL, which contains committed granule cell precursors, and the ventricular zone, which contains multipotent stem cells that give rise to most cerebellar neurons. Several cancer predisposition syndromes carry an increased risk of medulloblastoma, including nevoid basal cell carcinoma syndrome or Gorlin syndrome, Turcot syndrome, and Li-Fraumeni syndrome. Gorlin syndrome, although rare, is an important factor that must be considered in the treatment of these patients, because they are at high risk for the development of nevoid basal cell carcinoma, especially within the involved radiation fields. In addition Gorlin syndrome provided the first clues as to potential pathways that may be involved in the pathogenesis of medulloblastoma, with the finding of mutations in SHH pathway members, such as PTCH1 , increase the risk of tumorigenesis.
Recent cooperative studies have comprehensively profiled the biology of medulloblastoma to reveal four distinct subgroups of tumors. The groups are distinguishable from each other on the basis of gene expression, methylation, copy number, and mutation profiles. These groups have been designated as Wnt-positive medulloblastoma, SHH-positive medulloblastoma, and group 3 and group 4 medulloblastoma. Wnt-positive medulloblastomas are characterized by mutations of β-catenin, resulting in activation of Wnt pathway signaling. Children with Wnt-positive medulloblastoma rarely have metastatic disease and have an excellent overall prognosis. The SHH group is characterized by tumors that carry mutations of the SHH pathway, with resultant pathway activation. The types of SHH pathway mutations differ between infants, children, and adults and may affect the use of targeted therapies for this group. These tumors can encompass multiple histologic subtypes, although nodular desmoplastic medulloblastomas are more likely to carry SHH pathway mutations. Group 3 medulloblastomas carry a poor prognosis and are largely characterized by amplification of MYC or amplification/overexpression of the OTX2 oncogene. Group 4 medulloblastomas carry an intermediate prognosis and are likely to have isochrome of 17q, and gene expression studies show enrichment of genes involved in neuronal differentiation and development. TP53 mutations have been described to be enriched in the SHH subgroup of medulloblastoma, and when present, they are associated with inferior responses to therapy. Profiling studies of recurrent medulloblastomas have revealed that the subgroup determinations are stable, with recurrent tumors maintaining the same subgroup profile as they were at diagnosis.
In addition to the driver genomic alterations identified in medulloblastoma, evidence is growing that epigenetic dysregulation may play a role in the pathogenesis of these tumors. This belief is supported by the observation of enrichment in genomic alterations of chromatin modifiers in large-scale profiling projects for medulloblastoma. These alterations include inactivating mutations of the histone methyltransferase genes MLL2 and MLL3, KDM1A a histone demethylase and SMARCA4, a member of the SWI/SNF chromatin remodeling complex. Mutations and aberrant hypermethylation of the telomerase reverse transcriptase (TERT) promoter have also been recently described in large-scale sequencing projects of medulloblastoma. Mutations in TERT promoters have been found to be particularly enriched in the SHH subtype of medulloblastoma. In addition altered expression of genes regulated by the polycomb repressor complex have also been described in medulloblastoma. Alterations in chromatin modifiers have been described in medulloblastoma, and novel agents that target chromatin modification are being investigated as potential therapeutic for medulloblastoma. These agents include drugs that suppress expression of the MYC isoforms by inhibiting bromodomain containing 4 (BRD4), a bromodomain and extraterminal (BET)–containing protein, and inhibitors of enhancer of zeste 2 (EZH2).
Alterations in genes involved in cell cycle regulation have also been described in medulloblastoma. These alterations include amplification of cyclin-dependent kinase (CDK)/cyclin D genes and loss of the cell cycle inhibitor p27Kip1. Inhibition of CDK6 has been shown to reduce cell proliferation in medulloblastoma cell lines, and small molecule CDK inhibitors may have a potential therapeutic role in this disease. In addition strategies that target G2-M regulators have also been investigated in model systems of SHH medulloblastoma using Aurora and Polo-like kinase inhibitors.
No specific single environmental factor has been demonstrated to be associated with the development of medulloblastoma, although the nonrandom occurrence of disease detected in children born in the fall raises the possibility that some environmental or infectious pathogen may be implicated. The association between the development of medulloblastoma and viruses remains controversial. Multiple reports implicating polyomaviruses (including JC and SV40 virus in animal models) as a causative agent in medulloblastoma have been published. Human medulloblastoma samples have been found to possess JC and SV40 viral sequences. However independent confirmation of JC and SV40 sequences in human tumors could not be duplicated. Prematurity was identified as a significant risk factor in one study. Whites are affected 42% more often than blacks. In contrast several genetic syndromes (e.g., nevoid basal cell carcinoma syndrome or Gorlin syndrome, Turcot syndrome, and Li-Fraumeni syndrome) are associated with a significantly increased risk for the development of medulloblastoma. Gorlin syndrome, although rare, is an important factor that must be considered in the treatment of these patients, because they are at high risk for the development of nevoid basal cell carcinoma, especially within the involved radiation fields. Children of African-American descent require especially careful skin evaluation for the early detection of these lesions.
Clinical Presentation
Many of the clinical manifestations of medulloblastoma are similar to those of other tumors that arise in the fourth ventricular region and are related to obstructive hydrocephalus. Headache, nausea, and vomiting, characteristically in the morning, are the most common initial symptoms and usually precede diagnosis by 4 to 8 weeks, although longer intervals are not uncommon, especially if the headaches and vomiting caused by obstructive hydrocephalus are intermittent. Interestingly it appears that the duration of presenting symptoms may correlate inversely with disease state at the time of presentation; patients with low-stage disease were shown in one series to have a longer median duration of symptoms than were patients with high-stage disease. Personality changes (irritability) are an early feature but may be difficult to recognize as a sign of a brain tumor. Other features that can lead to diagnosis include lethargy, diplopia, head tilt, and truncal ataxia. The common signs found on physical examination are papilledema, ataxia, dysmetria, and cranial nerve involvement. Abducens nerve (cranial nerve VI) palsy as a result of raised ICP may cause diplopia and head tilt. Torticollis can be a sign of cerebellar tonsil herniation. Less commonly, intratumoral hemorrhage may lead to acute onset of confusion, headache, and loss of consciousness. The presentation in infants may include an abnormal rate of increase in head circumference. Other presenting symptoms can include loss of previously achieved milestones and failure to thrive. With regard to metastatic disease, at the time of diagnosis, although lumbar CSF analysis or MRI-visualized leptomeningeal metastases occur in 20% to 30% of children with medulloblastoma, clinical manifestations of metastases are uncommon. Back pain and radicular pain may indicate the rare complication of spinal canal dissemination. Attention to the clinical manifestation of posterior fossa tumors is important because the presenting signs mimic many other common ailments in children, including viral infections and stress. Because tumors have a significant propensity to metastasize early, careful evaluation of associated neurologic signs (e.g., papilledema and diplopia) can help identify children in need of referral for imaging sooner.
Imaging and Histology
Most medulloblastomas arise in the cerebellar vermis and extend into the fourth ventricle, resulting in obstructive hydrocephalus. The remainder are localized to the cerebellar hemisphere, especially in older patients and those with the desmoplastic subtype. The signs and symptoms of obstructive hydrocephalus, such as severe headache, morning vomiting, long tract signs, and papilledema, can be a medical emergency and require immediate evaluation. CT scanning of the head or rapid sequence MRI is the immediate choice of imaging to rule out an obstructive mass. This rapid study provides good differentiation between the fluid cavities and brain and will be sufficient to allow the neurosurgeon to determine whether immediate action is required. Often CSF diversion can be avoided if tumor resection is immediately possible. When required third ventriculostomy is preferred to VP shunts. All patients will require high-quality MRI scans of the brain and spine, including the entire thecal sac at the base of the spine. Lumbar puncture for CSF cytologic examination in newly diagnosed patients should be deferred if any possibility of elevated ICP exists; lumbar puncture is usually better performed after the tumor has been resected and CSF flow has been restored.
The MRI findings may differentiate between medulloblastoma types, as well as from other cerebellar tumors, such as ependymoma and pilocytic astrocytoma, and may be helpful before surgical resection. However atypical appearances of these three tumors can also occur, and thus a diagnosis based solely on imaging characteristics currently is not feasible. The appearance of a vermian location, hypercellularity (often demonstrated as dark areas of tumor on T2-weighted imaging), and intratumoral hemorrhage is more compatible with a cellular medulloblastoma or an ependymoma. A tumor that fills the fourth ventricle and extends through the foramina of Luschka and Magendie is more likely to be an ependymoma, although one with a large cyst and mural nodule (with a small area of solid tumor) is more consistent with a PA. PAs typically arise in the cerebellar hemisphere and are imaged on T2-weighted MRI as areas of homogeneous high-signal intensity, with the fluid collections defining the less intense tissue components of the tumor.
Medulloblastoma has T1-weighted MRI signal characteristics more similar to those of gray matter, reflecting hypercellularity and typically resulting in a relatively homogeneous image. On T2-weighted images these tumors can appear to be hyperintense or, more frequently, they can display mixed signal characteristics indicative of small intratumoral cysts, calcification, or small areas of hemorrhage. The background signal intensity of medulloblastoma on T2-weighted images is characteristically lower than in other tumor types, indicating a dense packing of cells and a high nuclear-to-cytoplasmic ratio. Because medulloblastoma typically arises in the roof of the fourth ventricle, a cleft of CSF beneath the tumor in the fourth ventricle helps distinguish this tumor from ependymoma. Tumors are typically enhanced with administration of gadolinium ( Fig. 57-36 ). The presence of characteristic gyriform morphology and a well-circumscribed appearance on an MRI scan suggest the presence of the nodular subtype of medulloblastoma. Functional imaging with PET and SPECT are helpful for the improved diagnosis of medulloblastoma. A number of agents such as oxidronate (OctreoScan) can directly assess for the presence and activity of certain cellular constituents, which helps differentiate recurrent disease from scarring.
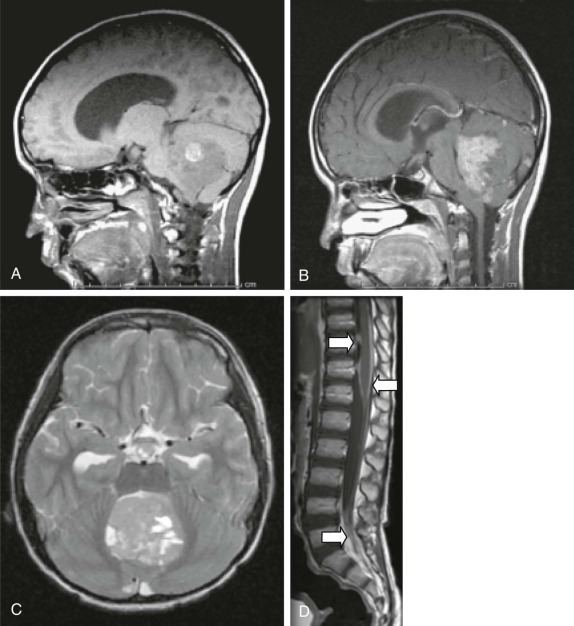
Histopathologic analysis of resected tumor specimens is essential for diagnosis and treatment planning. It should be noted that the histopathologic classification and nomenclature of medulloblastoma, within the broader context of embryonal tumors, has long been controversial and even today shows signs of continued evolution. The most current WHO classification schema considers medulloblastoma to be an independent entity in the group of embryonal tumors, separate from sPNET (now called CNS PNET in the revised WHO classification ). Within medulloblastoma, it is also clear that these tumors are not a homogeneous entity from a histopathologic viewpoint. At present five main histologic subtypes are recognized—classic, desmoplastic-nodular, anaplastic, large cell, and medulloblastoma with extensive nodularity. In addition any of these five variants of medulloblastoma can have areas of myogenic or melanotic differentiation. Classic (nondesmoplastic) medulloblastoma is the most common subtype, with approximately two thirds of tumors being classified as such, followed by the desmoplastic subtype (25%). This distribution is not uniform and is influenced by factors such as age and the presence of Gorlin syndrome. Individual tumors are often heterogeneous with regard to the extent of desmoplasia.
Classic medulloblastoma consists of uniform sheets of densely packed small round blue cells with round to oval hyperchromatic nuclei and scant cytoplasm ( Fig. 57-37 ). Desmoplastic medulloblastoma is characterized by so-called pale islands, or reticulin-free nodules, that are surrounded by reticulin-producing proliferating cells. Desmoplastic medulloblastoma is linked to the nevoid basal cell carcinoma (Gorlin) syndrome, caused by mutations in the PTCH1 gene and leading to dysregulation of SHH signaling. Only recently have the large-cell and anaplastic variants of medulloblastoma been identified. These variants are defined by light microscopic findings of prominent nuclei with a high degree of pleomorphism, high mitotic indices, increased cytoplasm, and a higher rate of necrosis than in other variants. These tumors are uniformly positive for synaptophysin and are generally positive for chromogranin. Anaplastic and large-cell medulloblastomas tend to have higher rates of metastasis at presentation, loss of chromosome 17p13.3, and MYC amplification, and both have relatively poor prognoses. These two variants can occur together and contain cells with markedly increased nuclear atypia and/or large nuclei with prominent nucleoli ( Fig. 57-38 ).
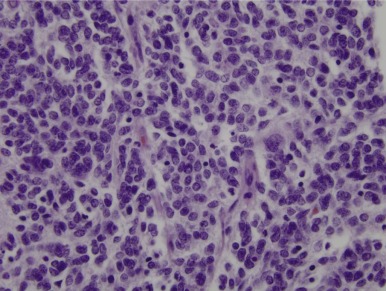
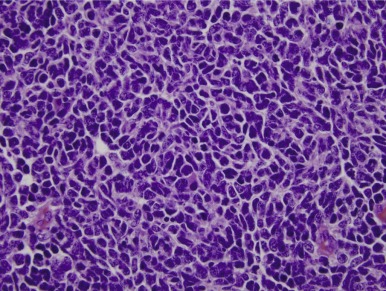
Wnt-positive medulloblastomas are usually classic histology. SHH group tumors include classic histology tumors, and nodular desmoplastic medulloblastomas most commonly belong in this group. Group 3 and 4 tumors have higher rates of MYC or MYCN amplification and isochrome 17q.
One of the cardinal features of medulloblastoma is its tendency to differentiate along one or more pathways, most commonly neuronal, astrocytic, and ependymal lineages. Synaptophysin expression, a characteristic feature of medulloblastomas, indicates neuronal lineage. The expression of certain intermediate filament proteins, such as nestin, vimentin, and GFAP, can also be seen in medulloblastomas. Medulloblastoma is divided into five variants:
- 1.
Nondesmoplastic (classic) medulloblastoma may be strongly immunoreactive for vimentin. Although GFAP expression is typically restricted to developing and mature astrocytes, most classic medulloblastomas contain GFAP-positive cells, which could indicate astrocytic differentiation.
- 2.
Nodular-desmoplastic medulloblastoma has been shown to have an increased degree of reticulin staining, with reticulin-free areas that can be identified throughout the lesion or only focally. The nodules represent regions of neuronal maturation. This rare subtype makes up only 5% of medulloblastomas but accounts for 57% of medulloblastomas in infants and accounts for the particularly good outcome in this age group.
- 3.
Anaplastic medulloblastoma has marked nuclear pleomorphism, a high mitotic rate, apoptotic cells, and significant atypia. If only small areas of anaplasia are identified, the tumor does not meet the diagnostic criteria for the anaplastic subtype. Experienced pediatric neuropathologists are therefore required for difficult cases. Anaplasia may be related to abnormalities in c-Myc .
- 4.
Large-cell medulloblastoma is a rare subtype with abundant mitoses and apoptotic bodies. The name derives from the large nuclei that are present. It often contains areas of anaplasia, possibly related to specific aberrant signaling pathways, and thus many refer to these two variants as large-cell/anaplastic medulloblastoma.
- 5.
Medulloblastoma with extensive nodularity, previously called cerebellar neuroblastoma, is typically identified in infants and has very large reticulin-free zones in comparison with desmoplastic medulloblastoma.
Medulloblastoma with myogenic differentiation refers to the presence of rhabdomyoblastic elements and can be seen with any of the five variants of medulloblastoma discussed earlier (classic, desmoplastic, anaplastic, large cell, and medulloblastoma with extensive nodularity). The historic term for medulloblastoma with rhabdomyoblastic elements was medullomyoblastoma , although this latter term is no longer recommended. Similarly medulloblastoma with melanotic differentiation refers to the presence of melanin and does not represent an additional unique variant of the disease. Rather melanotic differentiation can occur with the other recognized variants. This tumor type was previously called “melanocytic medulloblastoma.”
Mitoses and other markers of proliferation such as the MIB1 labeling index are common features of these tumors and do not alter the prognosis. A mitotic index of 0.5% to 2%, an MIB1 index higher than 20%, and the presence of Homer-Wright rosettes, that is, cells arranged around a central lumen or hub ( Fig. 57-39 ), are often associated with increased atypia and mitotic activity and are seen in approximately one third of cases. Pseudorosettes—that is, cells arranged around a central clearing with a vessel in the middle—are a common feature of medulloblastoma but are variable; they are not prognostic. Vascular proliferation and necrosis are uncommon.
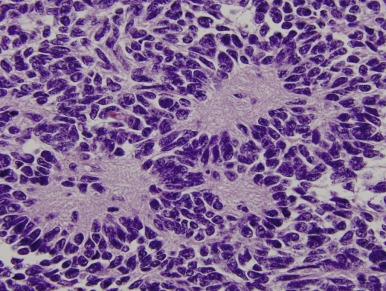
Determining the cellular origin of medulloblastoma may be a key to the proper stratification and management of these tumors. As noted earlier current evidence indicates that patients with the desmoplastic variant, which is thought to originate from cerebellar granule cell precursors, have a better prognosis than patients with the classic or large-cell/anaplastic variants. Similarly patients with the large-cell and anaplastic variants appear to have a worse prognosis, and our developing understanding of the unique pathways involved will likely allow better classification and therapy in future studies.
Historic Perspective
Like most other treatments, the development of our current approaches to multimodality therapy for medulloblastoma is the result of a series of overlapping clinical trials. It is beyond the scope of this chapter to give a detailed recounting of the progression to our current understanding of medulloblastoma therapy. Rather a few key events will provide some idea of why we do what we currently do and indicate some of the weaknesses on which our current assumptions are based.
For decades after the first description of medulloblastoma was published by Bail and Cushing in 1925, the prognosis for this highly malignant tumor was extremely poor. Cushing’s development of neurosurgical techniques to remove tumors of the fourth ventricle, coupled with a dramatic decline in surgical morbidity, was critical to the development of effective therapies. With the introduction of radiation therapy and further refinements in neurosurgical techniques between 1950 and 1970, significant improvements in survivorship were achieved. For the first time patients with standard-risk disease had a 5-year EFS approaching 50%, whereas those with high-risk disease were estimated to have a 5-year EFS of approximately 20%. It was demonstrated that radiation therapy could cure a significant percentage of patients, thus securing its place in the standard approach for these tumors. The craniospinal dose for all patients at that time was 3600 cGy (craniospinal irradiation; CSI), with a posterior fossa boost to an approximate total of 5400 cGy. The promise of EFS was tempered, however, by the poor functioning levels seen in surviving patients.
Based on these outcomes and late effects, treatments were modified on the basis of risk categories. For high-risk patients, chemotherapy was added to full-dose 3600 cGy craniospinal radiotherapy, and the resulting improvement in outcome was easily measured, with 3- and 5-year EFS rates of approximately 60%. By contrast the approach to patients with standard-risk disease was to reduce the dose of craniospinal radiotherapy from 3600 to 2400 cGy to spare neurocognitive function, while maintaining the boost to the posterior fossa of 5400 cGy. A national clinical trial in the United States was undertaken, but as the study progressed, there was concern that the number of recurrences in the 2400-cGy treatment arm was greater than that for the 3600-cGy treatment arm. Consequently the study was terminated early. With further follow-up, however, the survival curves for the two treatment arms began to approach one another, such that no statistical difference in outcome was identified, and the early termination of the trial hindered the determination of the statistical conclusion. The results from these treatment studies suggest that chemotherapy in combination with craniospinal radiation therapy could positively affect outcome. The strategy for standard-risk disease thus became the lower-dose radiation therapy of 2400 cGy CSI with the addition of chemotherapy. In follow-up studies of these combinations, PFS of 86% and 79% at 3 and 5 years, respectively, was achieved for standard-risk patients. The two approaches for high- and standard-risk disease have now become the basis of most North American treatment trials.
Current Treatment
Management.
A high level of clinical suspicion is critical for an early diagnosis of medulloblastoma. Neuroimaging is usually the first step, with CT or MRI scanning of the brain frequently performed in the acute setting. MRI of the brain and spine should be performed as soon as is feasible. In children in whom the diagnosis of medulloblastoma is suspected, an MRI of the spine should be obtained because the incidence of CSF dissemination at diagnosis ranges between 20% and 30%. Corticosteroids are usually used to control increased ICP. A lumbar puncture should be deferred until after intracranial hypertension has been relieved by surgery. Because extraneural spread of medulloblastoma is possible, bone marrow aspiration and biopsy should be considered for the complete evaluation of patients with medulloblastoma, although these procedures are no longer required for participation in cooperative clinical trials. Although the exact incidence of extraneural metastases is evolving, current estimates suggest that such metastases occur in fewer than 5% of patients. Because patients will require general anesthesia for insertion of a central line for the administration of chemotherapy, bone marrow samples and a baseline lumbar sample can be easily obtained with minimal trauma to the child or family. CSF obtained from the ventricular or cisternal fluid is not considered an adequate sample upon which to base staging decisions; thus lumbar sampling is recommended for all patients in whom such sampling is not contraindicated pretherapy.
At present risk stratification and treatment assignment are based primarily on clinical factors, although this will change with greater reliance on molecular profiling. Recently histology has been added in the risk assignment of medulloblastoma. The modified Chang criteria are based on the extent of tumor and the degree of metastasis ( Box 57-3 ). After completion of the staging evaluation (i.e., MRI of the brain and spine, CSF cytology, and bone marrow studies), histology, and degree of resection, patients are stratified to the standard-risk, high-risk, or infant-risk groups. The major determinants of clinical risk categorization are age at diagnosis (younger than 3 years vs. 3 years or older), metastasis or M stage (M0 versus higher than M0), volume of residual postoperative disease (less than 1.5 cm 3 residual disease vs. greater than 1.5 cm 3 residual disease), and histology (anaplastic–large cell versus other). Although these criteria are widely used in the staging of medulloblastoma, the role of complete resection versus less than 1.5 cm 3 residual disease, the age of the patient, and the significance of anaplastic histology are still in question, and entry criteria may vary from those indicated.
Stage M0: No evidence of subarachnoid or hematogenous metastases
Stage M1: Microscopic tumor cells found in the cerebrospinal fluid
Stage M2: Gross nodular metastatic seeding in the subarachnoid space or ventricular system distant from the primary site of disease
Stage M3a: Gross nodular seeding in the spine subarachnoid space without evidence of intracranial seeding
Stage M3b: Gross nodular seeding in the spinal subarachnoid space, as well as intracranial seeding
Stage M4: Extraneural metastases
Histology has just recently begun to be considered in risk stratification. Most prior published series of patients with medulloblastoma had incorporated patients with anaplasia–large cell medulloblastoma into standard-risk treatment if they met the other criteria for standard risk. The recognition that infants with the desmoplastic variant may do better than infants with the nondesmoplastic variant may provide an opportunity to deescalate therapy in this particular group. Newer protocols are now incorporating these variables. Tolerability of therapy has also recently come to light as more adult programs begin to treat older patients with regimens similar to those that have produced significant advances in pediatric patients. Older patients, including adolescents, do not tolerate therapy as well as younger children or infants, which provides an argument for dose modifications in this group.
ATRTs of the posterior fossa often resemble medulloblastomas. In prior cooperative group studies, up to 30% of patients with ATRTs were misclassified as having a medulloblastoma. With the use of INI1 immunohistochemical analysis of the tumor sample, rapid differentiation of these two histologies is required because the treatment for ATRT differs from that of medulloblastoma in most large cooperative groups.
Treatment
Surgery.
Treatment of medulloblastoma, as well as of other embryonal tumors, is multimodal, consisting of surgery, radiation therapy, and chemotherapy, with current therapies guided primarily by age and stage at diagnosis. The initial goal of surgery is to control raised ICP, if present. Once the safety of the patient is ensured, consideration of tumor resection becomes the next objective. If metastatic tumor is already known to be present, immediate aggressive surgery within the posterior fossa will not reduce the need for more intensive radiation therapy and chemotherapy. Rather the goal of surgical resection should be to achieve maximal tumor volume reduction without significant damage to adjacent areas. In balance it is more important for the neurosurgeon to preserve function rather than to maximize resection. Given the excellent prognosis for patients with medulloblastoma, even with metastatic disease, damage to the brain in a high percentage of patients means a high percentage of survivors with permanent neurologic damage. A third ventriculostomy (preferred) or VP shunt should be deferred prior to initial surgery but may be necessary if sufficient resection to reopen CSF flow is not achieved.
A number of surgical approaches are possible to optimize resection of posterior fossa and cerebellar tumors. Most fourth ventricular lesions will require interruption of the cerebellar vermis, although resections through the vermis using horizontal or vertical incisions have both been associated with posterior fossa syndrome. Even attempts at going under rather than through the vermis have been associated with this morbidity. The brainstem must also be protected while removing medulloblastomas within the fourth ventricle. Because these tumors can invade the brainstem, intraoperative decisions regarding the aggressiveness of resection must be made. Similarly resection must protect the nearby cranial nerves. New neurosurgical techniques are now available that allow for greater guidance in resection and in physiologic monitoring so that damage to important anatomic structures can be avoided. The use of intraoperative MRIs has improved the neurosurgeon’s ability to ensure adequate yet safe resection.
Potential complications of surgery in this location, regardless of tumor type, include cerebellar mutism (also called posterior fossa syndrome) and aseptic meningitis. The posterior fossa syndrome, or cerebellar mutism, occurs in approximately 10% to 20% of cases, although it is being reported in an increasing percentage of patients as more attention is given to the quality of survivorship. The characteristics are reduced speech output or mutism, personality changes, hypotonia, ataxia, and reduced oral intake. Symptoms typically appear 1 or 2 days after surgery, may range between mild to severe, and may last from days to months with varying degrees of recovery.
Radiation Therapy.
Radiotherapy has become an important modality in the long-term outcome of patients with medulloblastoma. During the past 20 years, a great deal of effort has been focused on the reduction of craniospinal doses as a result of significant long-term neurocognitive damage, as well as secondary tumor risk and stroke. Radiotherapy is currently risk adapted, and additional modifications to dose and volume continue. For standard-risk patients, 5400 to 5580 cGy are administered to the tumor bed. This volume continues to evolve, from inclusion of the entire posterior fossa, even when the tumor is small and focal, to more limited fields that encompass only structures in contact with the tumor and a small margin. Previous standard-radiation therapy included craniospinal doses of 3600 cGy. A national clinical trial evaluating standard-risk patients with a craniospinal dose of 2400 cGy demonstrated equal efficacy to historic reports, although this study did not randomize between the two CSI doses; 2400 cGy has been accepted as the new standard for this patient population. An important conclusion from this study was the poor outcome of patients with high-risk disease treated with lower dose craniospinal radiotherapy, reinforcing the importance of proper staging and radiation planning for all patients. A COG clinical trial for patients with standard-risk medulloblastoma is ongoing; this trial randomizes children younger than 8 years to receive either 2400 or 1800 cGy CSI and randomizes all children to receive either a full posterior fossa boost or an involved field boost. This trial will help define the dose and field of radiation therapy required for this population.
For patients with high-risk disease that is caused either by the presence of bulk unresectable disease within the posterior fossa or by the presence of metastatic disease, treatment remains 3600 cGy to the craniospinal axis and a focal dose to the involved posterior fossa of 5400 to 5940 cGy. Although this dose to the entire brain and spine increases the morbidity of therapy, it is an important component of effective treatment, as noted in the previously described randomized trial for standard-risk patients who, on central review, were found to have high-risk disease. A number of cooperative group trials are currently evaluating drugs combined with radiotherapy (radiation sensitizers) to augment the activity of radiation therapy in children with high-risk medulloblastoma.
Infants with medulloblastoma (and other CNS PNETs) continue to pose the greatest therapeutic dilemma. Even in the presence of standard-risk disease, the contraindication for craniospinal radiation therapy limits the treatment options for this group and their long-term prognoses. The omission of craniospinal radiotherapy has required the acceptance of a lower cure rate but a much higher functionality for those who do survive. The use of treatment protocols that rely only on surgery and chemotherapy demonstrate a relatively poor outcome for this group in all but a select group (e.g., those with nodular desmoplastic medulloblastoma ), again supporting the importance of radiation therapy for the treatment of medulloblastoma. Because many relapses occur locally, many centers have tested focal radiotherapy to the posterior fossa, forgoing the craniospinal component of therapy. This approach attempts to improve local control to the cerebellum, which is thought to be an area less important for neurocognitive development than the cortex. However in a recently completed COG study (trial P9934) that pilot-tested focal radiation therapy to the posterior fossa, it was noted that many recurrences occurred in sites that were outside the radiation field.
Hyperfractionation of radiotherapy—that is, twice-daily dosing of radiation therapy—has not demonstrated significant improvement in outcome, although one pilot trial has reported such an improvement. By contrast a significant delay in the completion of the radiation therapy appears to negatively affect survival. Proton radiation therapy is increasingly used for children with medulloblastoma. Although its efficacy is similar to that of conventional photon radiotherapy, the absence of an exit dose can reduce radiation exposure to areas uninvolved with tumor. The limited number of proton beam facilities, however, restricts the wider application of this technique. Other more readily available radiation therapy options include IMRT. In particular, tighter margins within the posterior fossa and better 3D conformal planning with IMRT-based techniques can help avoid the auditory apparatus and thus long-term hearing impairment.
The potential use of chemotherapy before delivery of craniospinal radiotherapy has been pilot tested and has been shown to be associated with inferior EFS and OS. A national clinical trial with randomization between standard-dose radiation therapy (3600 cGy) and reduced-dose (2340 cGy) CSI in patients with low-stage (M0) disease who were not receiving adjuvant chemotherapy resulted in an increased rate of recurrence outside of the posterior fossa. However it should be noted that no statistically significant difference was found between the two groups at 6 to 7 years after treatment.
Chemotherapy.
The chemosensitivity of medulloblastoma and the benefits of adjuvant chemotherapy in treatment regimens have been demonstrated by a number of studies. During the past 20 years studies have shown a nearly 20% to 30% improvement in EFS and OS by using chemotherapy during and/or after radiation therapy. Most regimens have included vincristine, cisplatin, etoposide, and an alkylator (cyclophosphamide or CCNU). Although platinum-containing regimens are considered important components of the treatment of medulloblastoma, the ultimate cumulative dose required for optimal activity while minimizing renal and audiologic impairment remains to be defined. Chemotherapy is currently a standard adjuvant therapy in children. Similarly infant studies with chemotherapy alone have shown the importance of chemotherapy as an effective strategy for patients with medulloblastoma. For most of these infant studies the removal of CSI has had a negative effect on survival, although a significant proportion of survivors can achieve cure. Recently methotrexate has been added into multiagent chemotherapy for medulloblastomas and other PNETs with excellent tumor response. In one phase II study consisting of four cycles of carboplatin, etoposide, and methotrexate, CR and partial response (PR) rates of 71% and 81% were achieved in patients younger than 3 years and older than 3 years, respectively.
The concurrent use of craniospinal radiation therapy and multiagent chemotherapy for medulloblastoma can result in significant impairment of nutrition during therapy for these patients. Patients who present with persistent vomiting likely initiate therapy in a negative nutritional state. A large proportion of patients will require nutritional support through a nasogastric tube or gastric tube. Constant surveillance of nutrition is important because this issue is often dismissed early and is only recognized when severe weight loss has occurred.
Impairment in hearing function continues to be a major concern in survivors of medulloblastoma. The overlap between posterior fossa radiation therapy and the use of ototoxic drugs such as cisplatin has resulted in significant toxicity for this patient population. Conformal radiation planning with tighter margins, the use of IMRT, and the use of proton beam radiation therapy can all significantly reduce the damage to the auditory apparatus. Recent medulloblastoma treatment protocols have also reduced the cumulative doses of cisplatin. The use of amifostine continues to be controversial, with some studies showing efficacy at protecting hearing and other studies not showing efficacy. Concerns about protecting the tumor from the chemotherapy remain, and only a well-controlled randomized trial will resolve this question.
Standard-Risk Disease.
The components of combination chemotherapy used during and after CSI continue to evolve. A large national clinical trial involving 2400 cGy CSI with a boost to 5400 cGy to the posterior fossa and randomizing between vincristine, cisplatin, and either CCNU or cyclophosphamide (COG trial A9961) demonstrated that both treatment arms have approximately similar activity. The OS for both groups was approximately 90%, with only a slight difference in EFS between the two chemotherapy regimens (85% for patients who received CCNU vs. 83% for those who received cyclophosphamide). The emphasis in the ongoing clinical study has been to reduce the CSI dose further in a randomized fashion for children younger than 8 years (i.e., those most at risk for the severe neurocognitive impact of radiation therapy) while combining the CCNU and cyclophosphamide agents to reduce the total exposure to each. The safety of 1800 cGy craniospinal radiotherapy was pilot tested in 10 patients with standard-risk medulloblastoma, and in this small cohort, the survival outcome appeared equally effective to that for 2400-cGy therapy and with less neurocognitive impairment. Radiation- and chemotherapy-adapted approaches are especially important for younger patients given the significant dose-dependent neurocognitive impact of radiation therapy in this population. A large European cooperative group trial of standard versus hyperfractionated radiation therapy showed no significant outcome difference between these two approaches and has led to the adoption of daily fractionated radiation therapy as the standard for children with medulloblastoma.
High-Risk Disease.
Treatment strategies for this population have focused on the addition of new agents, including radiosensitizing agents and novel biologic inhibitors, targeting pathways important for these tumors in the hopes of further improving outcome. It should be noted that the sequence of treatment has been shown to be of major importance, with the best survival rates being achieved if radiation therapy is not delayed, although a recent study (Pediatric Oncology Group [POG] trial 9031) reported no difference in EFS in children who received radiation therapy first compared with those who received chemotherapy first in the presence of high-risk disease.
Infant Chemotherapy.
Patients younger than 3 years at diagnosis are considered to have higher risk disease at all stages and degrees of resection, related to the limitations of delivering craniospinal radiation therapy to this group. There is also an increased frequency of leptomeningeal dissemination at the time of diagnosis in young children (27% to 43%) versus older children (20% to 25%) with similar histologic diagnoses. After surgical resection, infants with medulloblastoma are often treated with chemotherapy alone or chemotherapy with involved field radiotherapy in an effort to reduce the high incidence of developmental and neuropsychological sequelae caused by craniospinal irradiation. It is clear that the risks of craniospinal radiation therapy in infants and young children are significantly greater than the benefits of therapeutic response in terms of neurocognitive development. Currently several chemotherapy regimens designed to delay or eliminate the need for whole-neuraxis radiation therapy are being investigated, including regimens using intraventricular chemotherapy. In addition high-dose regimens have been found to be feasible and effective for young children with disseminated medulloblastoma. The relative merits and risks of conventional multiagent chemotherapy compared with high-dose submyeloablative or myeloablative chemotherapy with stem cell support is under investigation. An important finding in a recent study for infants with medulloblastoma is the particularly good outcome in infants with the desmoplastic variant, even without radiation therapy, suggesting that nodular desmoplastic medulloblastoma may represent a low-risk group that does well with less intensive therapy. These tumors are also commonly of the SHH subtype, and it is possible that integration of novel agents targeting this pathway may have a potential therapeutic role. Ongoing studies to confirm this finding are under way but raise the possibility that desmoplastic medulloblastoma may form a new low-risk group.
Prognosis
Patients with standard-risk medulloblastoma have a 5-year survival of approximately 75% to 90%, whereas 5-year survival in patients with high-risk medulloblastoma remains at between 40% and 60%. Infants with metastatic disease or bulky unresectable disease continue to do poorly, with a 3-year EFS of less than 40%, except in those with the desmoplastic variant. Patients with other rarer subtypes of medulloblastoma, including the large-cell, anaplastic, large-cell/anaplastic, and melanotic variants, continue to do poorly, even with maximal upfront therapy. Various histologic features associated with improved prognosis have been evaluated, of which nodular appearance appears to be most important. The presence of dissemination is the single most important factor that correlates with poor outcome. Lifelong repeated surveillance imaging is required in anticipation of radiation-induced secondary malignancies, such as a meningioma or HGG. Radiotherapy, particularly in young children, can also cause significant adverse late effects in neurocognitive development, growth, and endocrine function. Gene profiling of medulloblastoma was an accurate method of predicting outcome, even when evaluated in the context of clinical data. Variables such as age, M stage, and degree of resection did not significantly improve the predictive ability of this independent data set.
Relapsed Medulloblastoma
Relapses occur as failures at the primary site, at distant sites, or both. MRI is not always accurate when lesions are identified, and pathologic correlation is important in avoiding toxic therapy for patients whose disease has not actually progressed. Tumors occurring at the primary site within the posterior fossa may have a greater chance for salvage. Patients treated without upfront radiation therapy (infants, in particular) also have a good salvage rate. Retrieval therapy is rarely curative, but long-term disease control may occur with high-dose myeloablative chemotherapy in a small proportion of patients. Past experience has shown that patients entering high-dose chemotherapy without chemotherapy-responsive disease, or with residual disease at the time of transplantation, are unlikely to benefit from this approach. A large number of new intrathecal, biologic, and antiangiogenic agents are being evaluated for patients with relapsed medulloblastoma. Although these agents are likely to be more effective when moved up early in the upfront setting, additional testing is still required. Most exciting are preliminary results with SHH inhibitors in this subgroup of patients. Given the distribution of mutations within the SHH pathway that differ between infants, children, and adults, as well as concerns for premature bone closure in infants and children taking these inhibitors, careful selection of patients and a balance of prognosis versus toxicity is required.
Future Directions
The recent discovery of genetic alterations defining different subtypes of medulloblastoma presents novel and exciting opportunities for the development of targeted therapies for children with medulloblastoma. Trials examining the efficacy of SHH inhibitors for those of the SHH subtype are currently under way, and novel agents that target the MYC transcription factor or activation pathways also are currently being investigated for MYC or MYCN amplified tumors. In addition studies that determine potential mechanisms by which medulloblastomas may acquire resistance to targeted agents are also important to provide insights into potential combination therapies. Conversely treatment strategies that minimize long-term therapy-related morbidity in children with Wnt-positive medulloblastoma are also being investigated. The challenge in the coming era will be to develop strategies that incorporate risk stratification and biologically targeted agents in the upfront treatment strategy for children with medulloblastoma.
Pineocytomas and Pineoblastomas
Tumors of the pineal region are often grouped together based on their location rather than on their cellular origins and behavior. Embryonal tumors of the pineal region include pineocytoma, pineal parenchymal tumor of intermediate differentiation (PPTID), pineoblastoma, and papillary tumor of the pineal region (PTPR). Together they account for less than 5% of all pediatric CNS tumors. Other pineal-based lesions include GCTs and, less commonly, astrocytic tumors. GCTs are discussed in a separate section. In a recent series of children diagnosed with pineal parenchymal tumors, the extent of surgical resection has been reported to be a significant prognosticator.
Pineocytomas
Pineocytomas arise from the pinocyte, the primary function of which appears related to photoreceptor activity and neuroendocrine function. They are classified as low-grade (grade I) neoplasms that are most common in adults and in late adolescence, although they can be observed in young children. They account for approximately 50% of the tumors of pineal origin, although pineal region neoplasms account for only 1% to 5% of all pediatric CNS tumors.
Clinical Presentation.
Like other pineal region lesions, pineocytomas often present with a constellation of symptoms related to their location. This constellation includes obstructive hydrocephalus and Parinaud syndrome, a cluster of abnormalities of eye movements and papillary dysfunction characterized by paralyzed upgaze, pseudo–Argyll Robertson pupils, convergence-retraction nystagmus, eyelid retraction, and a conjugate downgaze “sunsetting” sign. Impairment of hypothalamic-pituitary, brainstem, and cerebellar function is also possible. Unlike malignant pineoblastomas, metastases are rare.
Imaging and Histology.
On neuroimaging, pineocytomas are typically small focal lesions that can contain cysts and/or calcifications; they are best seen by CT. They are similar to other low-grade tumors on MRI, with strong contrast enhancement, hypointensity on T1-weighted sequences, and high signal intensity on T2-weighted sequences. Surgically these tumors tend to be well demarcated from surrounding tissue. Areas of hemorrhage are not uncommon. Microscopically pineocytomas are made up of small, round, mature-looking cells that have maintained features of pinocytes, including rosettes. These tumors demonstrate characteristic pineocytomatous rosettes that differentiate them from pineoblastomas. Mitoses are rare, as are other features of malignancy. Pineocytomas are usually strongly synaptophysin and neuron-specific enolase (NSE)–positive. If areas of pineoblastoma are identified with a pineocytoma, treatment is dictated by the most malignant element (i.e., pineoblastoma).
Management.
Surgery remains the mainstay of the therapeutic approach for these lesions. Treatment directed at the hydrocephalus with third ventriculostomy may provide an opportunity to obtain a biopsy before aggressive and potentially morbid surgical resection is attempted. Tumors with persistent growth can be irradiated, although the benefit of this treatment has not been clearly demonstrated. A role for chemotherapy has not been defined. Patients with incompletely resected tumors do not require therapy unless clear progression is demonstrated. Even patients with metastatic disease can remain untreated. The overall benign course observed with pineocytomas is in stark contrast to that of pineoblastomas.
Prognosis.
The long-term prognosis remains excellent, even with incompletely resected disease. Conservative management should be followed with regard to attempted surgical resection and radiation therapy. Because almost all patients with pineocytomas will be long-term survivors, the need to avoid toxicity is paramount.
Pineal Parenchymal Tumor of Intermediate Differentiation
PPTIDs are tumors of the pineal region that occur at all ages, although they are frequent in middle age, and are classified as grade II or III, depending on pathologic features. They account for 20% of pineal region tumors and were formally identified in 1993. Although reports are limited, there appears to be a slight female preponderance. Genetic evaluation has demonstrated a large number of abnormalities.
Clinical Presentation.
The presentation of patients with PPTID is similar to that of pineocytomas and includes obstructive hydrocephalus, as well as Parinaud syndrome, a cluster of abnormalities of eye movements and papillary dysfunction characterized by paralyzed upgaze, pseudo–Argyll Robertson pupils, convergence-retraction nystagmus, eyelid retraction, and conjugate downgaze. Impairment of hypothalamic-pituitary, brainstem, and cerebellar function is also possible. Unlike pineocytomas, metastatic disease appears to be slightly more frequent.
Imaging and Histology.
Imaging characteristics are similar to those of other lesions of pineal origin. They are cellular lesions with moderate nuclear atypia and a low mitotic index. Because of sampling error, diagnosis can be difficult with limited tissue, especially tissue obtained via third ventriculostomy.
Management.
Because of the increasing degree of malignant potential when compared with pineocytomas, attempts at surgical resection should be considered. Although formal pediatric studies are lacking, radiation therapy and chemotherapy should be considered. Intensity of treatment can be based on the localization of the tumor (focal, invasive, or disseminated), as well as the proportion of mitoses, necrosis, atypia, and possibly neurofilament protein expression.
Prognosis.
A definite prognosis for PPTID has not been clearly established. Grade is likely to play an important part in the long-term outcome of patients, as is the size and metastatic stage of patients. Because these tumors span all age groups, large homogeneous studies are difficult to perform.
Pineoblastoma
Unlike pineocytomas, pineoblastomas are highly malignant embryonal tumors. Like other small round blue cell tumors, these lesions are considered WHO grade IV and have a high propensity to metastasize. Typically they are observed in children younger than those seen with pineocytomas, although considerable overlap exists. Pineoblastomas can be associated with the genetic form of retinoblastoma and in such cases are often referred to as trilateral retinoblastomas. In a recent study of 408 patients with retinoblastoma, pineoblastoma was detected in 1% of all patients. An association with familial adenomatous polyposis has also been reported. Little is known about the molecular classification of these tumors with respect to their genesis or their association with other neural tumors of the CNS (PNET or medulloblastoma). Defects in TP53 have not been reported.
Clinical Presentation.
Presenting symptoms of pineoblastoma are virtually identical to those for pineocytomas, although the duration of symptoms may be shorter than that seen for other tumors in this location. Pineoblastomas often present with a constellation of symptoms related to their location, including obstructive hydrocephalus in most patients, as well as Parinaud syndrome, a cluster of abnormalities of eye movements and papillary dysfunction, as previously described. Impairment of hypothalamic-pituitary, brainstem, and cerebellar function is also possible. Pineoblastomas have a much greater propensity to metastasize and thus may demonstrate symptoms beyond the pineal region.
Imaging and Histology.
The neuroimaging characteristics of pineoblastomas are not pathognomonic for these tumors, but they are differentiated from pineocytomas by a significant increase in the volume of the tumor and the presence of much less distinct boundaries. MRI characteristics include low signal on T1-weighted sequences and heterogeneous areas of contrast enhancement ( Fig. 57-40 ). T2-weighted signals may be lower than in pineocytomas because of the higher nuclear-to-cytoplasmic ratio evident in these more malignant tumors. On histologic analysis these tumors are similar to other small round blue cell tumors, composed of sheets of cells but lacking the characteristic pineocytomatous rosettes of pineocytomas. Pineoblastomas are typically synaptophysin- and NSE-positive and other markers, including those for photoreceptor pathways, can be present, consistent with the developmental role of the pineal gland. Homer-Wright rosettes (i.e., cells with a central zone of cytoplasm; see Fig. 57-39 ) and Flexner-Wintersteiner rosettes (i.e., cells with a central zone of cytoplasm and a central space; Fig. 57-41 ) are common, but these rosettes can be seen in a large number of other embryonal tumors. These tumors often have significant atypia, and mitotic activity can be high. When additional pathways of differentiation are present (e.g., melanin, cartilage, and muscle), the tumors are called pineal anlage tumors. Regions of pineoblastoma can also be identified as part of a pineocytoma and are treated as a pineoblastoma. Although sporadic pineoblastoma has no clear genetic association, patients with hereditary (bilateral) retinoblastoma have a significant incidence of pineoblastoma (trilateral retinoblastoma). The clinical course of retinoblastoma-associated pineoblastoma can differ from that of sporadic pineoblastoma.
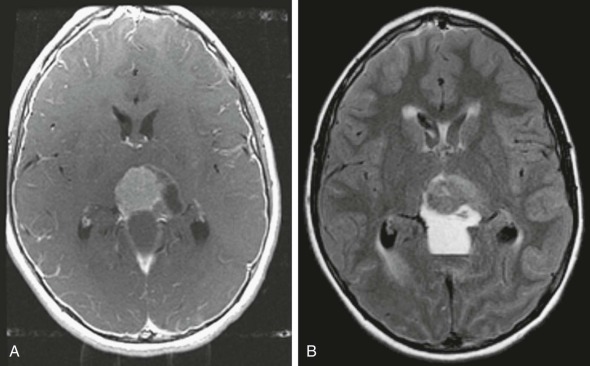
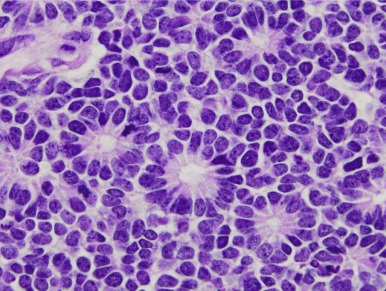
Recent profiling of genomic alterations have reported copy number alterations observed in pineoblastoma. These alterations include loss of chromosome 16q and amplification of PCDHGA3, and FAM129A. In addition DNA copy number profiles have reported more similarities between sPNETs and pineoblastoma than between these tumors and the lower grade tumors of the pineal region. The development of pineoblastoma has also been reported in a young patient with a germline mutation of DICER1 , which is also associated with the development of pleuropulmonary blastoma.
Management.
The therapeutic approaches for pineoblastoma have followed those of other embryonal seeding tumors of the CNS. The workup of patients with pineoblastoma includes immediate intervention for obstructive hydrocephalus and assessment of risk for herniation. A rapid CT or MRI scan, in conjunction with the examination and presenting history, will permit the neurosurgeon to assess these risks. If urgent CSF shunting is not required, then completion of the imaging baseline workup, including MRI of the brain and spine, can be performed and surgical resection can be planned. Unlike medulloblastoma, the location of pineoblastomas with adjacent vascular structures makes exploratory surgery much more risky, and referral to a highly specialized center should be considered. A third ventriculostomy or VP shunt should be deferred prior to initial surgery but may be necessary if sufficient resection with reestablishment of CSF flow is not achieved. In many patients an endoscopic biopsy of the tumor to confirm the diagnosis and concurrent third ventriculostomy can be performed with a planned open craniotomy and resection once the patient is clinically stable, the disease has been defined, and possible presurgical chemotherapy has been provided. Patients with metastatic disease at diagnosis should not undergo upfront aggressive surgery because adjuvant therapy will be required. For patients with focal disease at diagnosis based on initial MRI and documentation of negative results of a lumbar puncture, complete removal of the tumor ultimately will be an important component of long-term prognosis but should not be undertaken at any costs. Consideration for staged surgeries (second- and even third-look approaches) may be equally effective without causing permanent neurologic damage.
As with other embryonal seeding tumors of the CNS, craniospinal radiotherapy is an important modality in treating microscopic metastatic disease at presentation. Unlike medulloblastoma, pineoblastomas do not have a standard-risk category. All patients require aggressive therapy, including a craniospinal dose of 3600 cGy and a focal boost to the primary site of 5400 to 5940 cGy. Sites of metastatic disease require radiation boosts as well. Even with focal resectable disease, the prognosis for pineoblastoma falls significantly below that of medulloblastoma. In persons with disseminated disease, especially diffuse leptomeningeal spread, and infants, for whom craniospinal radiotherapy is contraindicated, the prognosis remains especially poor. The use of SRS or other similar modalities to deliver single-fraction ablative radiation therapy may provide added disease control of unresectable tumor, although these approaches are limited to small areas of disease.
Chemotherapy is an important modality for patients with pineoblastoma, and significant responses to chemotherapy have been observed, including in patients with trilateral retinoblastoma. Results from infant studies, which deferred radiotherapy, demonstrated that although chemotherapy can be effective at reducing bulk disease, few patients are long-term survivors with this modality alone. Because of the rarity of this entity most protocols have combined these patients with patients who have other embryonal tumors, including CNS PNETs and, occasionally, medulloblastoma. Specific estimates of survival from different therapies have therefore been difficult to determine. Current approaches continue to include initial craniospinal radiotherapy in combination with chemotherapy, followed by postradiation chemotherapy. Most regimens have used combinations of vincristine, cyclophosphamide, cisplatin, and etoposide. Because of the chemoresponsiveness of these tumors, pilot clinical trials with high-dose chemotherapy with stem cell transplantation have also been undertaken. The results of the German HIT2000 clinical trial, which included children with CNS PNET and pineoblastoma, recently reported 5-year EFS and OS of 24% ± 10% and 40% ± 12%, respectively, with multiagent chemotherapy and radiation therapy. Children with high-risk disease received intensified therapy.
The concurrent use of craniospinal radiation therapy and multiagent chemotherapy for pineoblastoma has resulted in a significant impairment of nutrition during therapy for these patients. A large number of patients will require nutritional support through a nasogastric tube or, more commonly, a gastric tube. Constant surveillance of nutrition is important because these issues are often dismissed early and are only recognized when severe weight loss has occurred. Patients with persistent vomiting usually initiate therapy in a negative nutritional state.
Prognosis.
The outcome of patients with pineoblastoma remains poor. Patients with focal disease that can be completely resected have 3- and 5-year EFS of approximately 50%. Infants, patients with incompletely resected disease by the end of therapy, or patients with metastatic disease continue to do poorly, with 3- and 5-year EFS estimates of 20% or lower. Progression tends to occur early and can occur at the primary site, throughout the CNS, or both. A recent study of recurrence patterns in children with embryonal intracranial tumors noted a high rate of relapses that involved the spine in children with pineoblastoma. Extracranial metastases are uncommon. Some patients with metastatic disease, however, are long-term survivors. After recurrence, no standard therapy has been documented to be effective, and most patients will succumb to their disease, usually within months.
Papillary Tumors of the Pineal Region
PTPRs are rare tumors of children and adults that were separated from other pineal tumors in 2003. As the name implies these neuroepithelial lesions possess papillary structures and are thought to be derived from specialized ependymal cells. Because of their varied appearance, they can be confused with ependymomas, choroid plexus tumors, and pineocytomas. PTPRs appear to fall into the WHO grade II or III category.
Clinical Presentation.
The clinical presentation of PTPRs is similar to that of other lesions of the pineal area. Hydrocephalus is present in almost all patients, and Parinaud syndrome is common. Impairment of hypothalamic-pituitary, brainstem, and cerebellar function is also possible. Metastatic disease does not appear to be as prevalent as in pineoblastomas.
Imaging and Histology.
PTPRs are well-circumscribed, often cystic lesions. They are hyperintense on T1- and T2-weighted sequences and enhance with administration of contrast material. Histologically these tumors stain strongly for keratins and are usually negative or weakly positive for GFAP (in contrast to ependymomas) and synaptophysin. Most of the tumor cells show strong expression of NSE, cytokeratins (particularly CK18), S-100 protein, and vimentin. The mitotic index and MIB1 labeling index are intermediate but of unclear prognostic significance.
Management.
In spite of their differentiated appearance, PTPRs are aggressive tumors that are not highly responsive to therapy. In a recent report of 31 patients, 21 of whom achieved a gross total resection, adjuvant radiation therapy alone resulted in a PFS rate of only 27%. The ability of achieve a gross total resection has been reported in some studies to be associated with improved PFS.
Prognosis.
In spite of complete resection and focal radiation therapy, local recurrences are common, although patients with complete removal of these tumors may do better. Dissemination appears rare, and craniospinal radiation therapy therefore does not appear to be required. Patients with an elevated MIB1 labeling index or mitotic rate may tend to do worse.
Supratentorial or Central Nervous System Primitive Neuroectodermal Tumors
Supratentorial or CNS PNETs are tumors of neuroepithelial origin. The classification of CNS PNET (previously called sPNET) as separate from medulloblastoma and other embryonal tumors, which was initially proposed in 1973, has not been without controversy. Historically these tumors have been called by various names, including cerebral medulloblastoma, cerebral neuroblastoma, and cerebral ganglioneuroblastoma. Rorke (1983) proposed that these tumors are the supratentorial equivalent of medulloblastoma, another common embryonal neoplasm. Although these tumors are histologically similar to medulloblastoma, they respond poorly to medulloblastoma-specific therapy. Part of the confusion in the approach to CNS PNETs has been in the nomenclature—the overlap in the term “PNET” for tumors of the body as well. These tumors do not share the molecular pathways, metastatic sites, or treatment responses of the extracranial PNET tumors. Even within the CNS, PNETs likely represent a heterogeneous group of tumors. According to the WHO classification schema, a number of subtypes of PNET are recognized: classic PNET, PNET with neuronal differentiation (called cerebral neuroblastoma), and, if ganglion cells are present as part of the tumor, cerebral ganglioneuroblastoma. Neither of the latter two entities is related to classic neuroblastoma, which is typically identified around the adrenal gland and shares a common name as a result of historical nomenclature only. New molecular determinants associated with PNETs of the brain have been identified using molecular and proteomic analysis and will, it is hoped, be useful for better subclassification of the diseases that fall into this broad category of tumors. Recently gene expression and genomic profiling efforts of CNS PNETs have confirmed that this disease entity is heterogenous and consists of multiple different tumor types. Copy number analyses of PNETs have in general revealed a complex karyotype with frequent copy number alterations. In addition these efforts have highlighted the inherent difficulty in classifying tumors based on histology. Molecular profiles have been found to match those of other tumor types, including ATRT (characterized by loss of INI1) and HGGs (characterized by the presence of mutations in the histone genes). Importantly, clustering of profiles have revealed that tumors have distinct profiles based on the underlying lineage of the tumor, including primitive neural, oligoneural, and mesenchymal lineages. These groups are associated with differences in clinical courses and prognosis, with tumors with an underlying primitive neural profile exhibiting the worse prognosis. Further, these profiling studies have shed light on other genes that may be involved in the pathogenesis of these brain tumors and thus may represent candidates for targeted therapies. These therapies include amplification of genes involved in cell cycle regulation.
CNS PNETs are relatively rare, occurring at a rate that is approximately 10% to 20% that of medulloblastoma. Precise statistics regarding incidence are difficult to ascertain because of historically different views regarding classification and nosology. These tumors are more common in early childhood, with most patients diagnosed before the age of 10 years, and white persons make up most of the reported cases. Most cases are identified in early childhood, but they span all ages. Although they are classified as supratentorial in nature, these tumors have been identified in the infratentorial and spinal compartments. A better term has recently been adopted by WHO — CNS PNET .
Clinical Presentation
Presenting features of CNS PNETs depend on the location of the lesion. Those adjacent to the ventricular flow path will usually include signs and symptoms of raised ICP as a result of obstructive hydrocephalus. Specific neurologic signs are dependent on the anatomic structures adjacent to the tumor and can include seizures, mood changes, or hemiparesis. Metastases are frequent in CNS PNETs, and clinical symptoms unrelated to the primary site of disease require investigation.
Imaging and Histology
CNS PNETs appear similar, independent of location within the CNS. As in medulloblastoma and pineoblastoma, these tumors can have areas of cyst or necrosis evident on CT or MRI. Lesions are dark on T1-weighted sequences unless hemorrhage is present and dark on T2-weighted sequences, reflecting their high nuclear-to-cytoplasmic ratio. CNS PNETs are usually contrast-enhancing after administration of gadolinium ( Fig. 57-42 ). Edema, as evident on T2-weighted or FLAIR sequences, is often prominent. All CNS PNETs are considered WHO grade IV. The light microscopic features of CNS PNETs can be similar to those of medulloblastoma or pineoblastoma. Tumors are characterized by the presence of neuroepithelial cells. Many CNS PNETs have evidence of differentiation along glial, neuronal, ependymal, or oligodendroglial lines, although most (approximately 60%) appear undifferentiated.
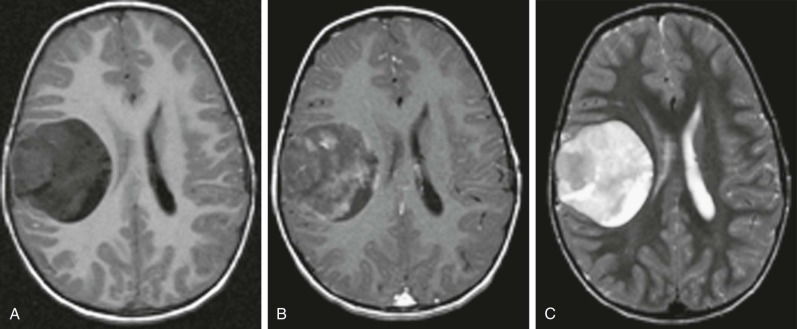
Immunohistochemical markers of CNS PNETs include synaptophysin, B-tubulin, and S-100. Occasionally GFAP can be identified in these tumors and indicates their ability to undergo divergent differentiation. Mitotic rates are often high and, although variable, the MIB1 labeling index is usually abundant. Mutations of p53 are rare and were identified in only 3 of 28 cases of CNS PNET in one study, although many tumors had overexpression of wild-type p53, which correlated with a poor prognosis. Specific markers for CNS PNETs that distinguish them from medulloblastoma do not exist. Other features, such as Homer-Wright rosettes and perivascular pseudorosettes (i.e., cells around a central blood vessel) are observed in many different tumors (see Figs. 57-35 and 57-39 ). ATRTs may resemble PNETs, but the presence of cells that stain positively for myosin but lack the protein produced by the SMARCB1 ( hSNF5/INI1) gene are diagnostic features for ATRT.
Medulloepitheliomas, Ependymoblastomas, and Embryonal Tumors with Abundant Neuropile and True Rosettes
Historically medulloepitheliomas, ependymoblastomas, and embryonal tumors with abundant neuropile and true rosettes (ETANTRs) have been considered distinct subtypes of CNS PNETs. However recent genomic and gene expression profiling efforts, including genome-wide DNA methylation and copy number profiling, have revealed similarities in genomic profiles, suggesting that they may represent spectrums of the same biologic entity. In this study, which included 97 tumor samples, all tumors were found to strongly express LIN28A, which had previously been described in ETANTRs. No difference in clinical features was observed, and unsupervised hierarchical clustering did not reveal subgroups for 41 tumors with sufficient material to perform the analysis, while as a group the tumors clearly separated from other pediatric brain tumors. Finally 95% of tumors were found to harbor amplification of chromosome 19q13.42, previously associated with embryonal tumors with multilayered rosettes. This study suggests that these entities share the same biology and can be considered spectrums of the same disease. However we will describe the traditional classification and clinical features of these tumors, in keeping with the current WHO classification.
CNS PNET tumors that have re-created features of the neural tube, called medulloepitheliomas, are typically found in very young children and have even been reported in newborns. They can occur throughout the brain, spine, and optic tract and can also occur outside the CNS. Their imaging characteristics differ somewhat from other CNS PNETs, with bright appearance on T2-weighted imaging. They tend to be large on macroscopic imaging. Additional immunohistochemical markers include nestin and vimentin staining. As with other CNS PNETs, maximal surgical resection, chemotherapy, and radiation therapy are the cornerstone of therapy. The predilection for young age in this subset of PNETs limits the use of craniospinal radiation.
PNET tumors with ependymoblastic rosettes are called ependymoblastomas and have features that overlap other CNS PNETs. These tumors predominate in infants and young children and can be found throughout the CNS, although most are associated with the ventricular system. Dissemination throughout the CNS is common, and symptoms include obstructive hydrocephalus, enlarging head circumference in infants, and focal neurologic deficits. The MRI appearance is similar to that of other PNETs, and edema is commonly observed. Treatment mirrors that of other CNS PNETs. Maximal resection is paramount, followed by multiagent chemotherapy. Although craniospinal radiation therapy is likely important for outcome, the prevalence of ependymoblastoma in infants precludes its use. Prognosis is poor, with rapid disease progression in most patients.
ETANTR is another rare CNS PNET tumor with a spectrum of histologic features and chromosomal abnormalities. These lesions can be large, cystic, and calcified and come to clinical presentation as a result of mass effect. Their rarity makes treatment recommendations difficult, although most are approached in a manner similar to that of other PNETs of the brain. The presence of isochrome 17 in only a few cases suggests that they are biologically distinct from medulloblastoma. Various cytogenetic abnormalities have been defined.
Cerebral Neuroblastomas
PNETs with neuronal differentiation are called cerebral neuroblastomas and are rare tumors of infancy and childhood. They are unrelated to the more prevalent peripheral sympathetic nervous system form of neuroblastoma and predominate in young infants, occurring in both the cerebrum and posterior fossa. Lack of immunohistochemical staining with IGF2 and IGF type 1 receptor, which is positive in many other infant CNS tumors, can help with the diagnosis.
A related entity of cerebral neuroblastoma is termed ganglioneuroblastoma , which has the presence of ganglion cells in addition to the neuronal component. These tumors cannot be differentiated from other malignant seeding tumors of infancy by MRI characteristics. Unlike most other CNS PNETs, cerebral neuroblastoma and CNS ganglioneuroblastoma may have a better prognosis with surgery and chemotherapy.
Treatment Strategies
A number of treatment protocols based on the successful therapeutic approaches of medulloblastoma have been applied to CNS PNETs. Repeated studies using maximal surgery, high-dose craniospinal radiotherapy to 3600 cGy with a boost to the primary site of at least 5400 cGy, and multiagent chemotherapy have not fared as well for CNS PNETs as for high-risk medulloblastoma, with 4-year survival of approximately 40% in one retrospective series. In one study of 55 patients treated with maximal surgery, craniospinal radiation therapy, and CCNU, vincristine, and prednisone versus 8-in-1 chemotherapy sandwiched in the middle, OS and EFS were comparable. The 3-year EFS was approximately 50% in the two groups and thus appeared similar to that reported in other studies. Poor prognostic variables included the presence of metastases and age younger than 2 years. Patients with pineoblastoma did better, with an estimated 3-year PFS of 65%. Compared with medulloblastoma, CNS PNETs often respond poorly to medulloblastoma-specific therapy, despite the fact that they are histologically similar. The use of high-dose chemotherapy with stem cell rescue has been successful for some young children in eliminating the need for radiation therapy, with 5-year EFS of 39%. The exact conditioning regimen and number of cycles of intensive chemotherapy remain under investigation. Recurrences are often disseminated, even when craniospinal radiation therapy is delivered upfront, and the salvage rate for these patients is exceedingly poor. In spite of these overall poor results, there have been reports of survivors after surgery, especially GTR, and radiation therapy. In one study patients treated with surgery, radiation therapy, and high dose stem cell–supported chemotherapy were reported to have a 5-year EFS of 75% ± 17% and an OS of 88% ± 13%. For patients with high-risk metastatic disease, the use of adjuvant carboplatin with radiation therapy as a radiosensitizer has been pilot tested in a phase I/II trial with promising results and is currently undergoing further investigation by the COG.
The concurrent use of craniospinal radiation therapy and multiagent chemotherapy for CNS PNETs has resulted in a significant impairment of nutrition during therapy for these patients. Therapy is intensive, and supportive care is important. A large number of children will require nutritional support through a nasogastric tube or, more commonly, a gastric tube. Constant surveillance of nutrition is important because these issues are often dismissed early and are only recognized when severe weight loss has occurred. Patients with persistent vomiting may initiate therapy in a negative nutritional state.
Prognosis
Although the prognosis of CNS PNET is well below that of medulloblastoma, 40% of patients will be long-term survivors. The most important variable for prolonged survival is the ability to achieve a complete resection in patients with nonmetastatic disease, followed by CSI and multiagent chemotherapy. The success of salvage therapy in patients who have had a relapse is exceedingly poor, even with high-dose chemotherapy and stem cell rescue.
Atypical Teratoid Rhabdoid Tumors
ATRTs are uncommon, highly malignant tumors seen primarily in infants and in young children, with a peak incidence between birth and 3 years. Cases throughout childhood and in adults have been reported, however, as has hereditary transmission of the susceptibility gene, resulting in congenital disease. Although CNS ATRTs account for approximately 1% to 2% of childhood brain tumors, they represent almost 10% of CNS tumors in infants. Rhabdoid tumors may arise anywhere in the body but are most common in the kidney, CNS, and soft tissues. CNS ATRTs are most commonly identified in the posterior fossa (60%) at the cerebellopontine angle, although supratentorial ATRTs are also frequently seen. Pineal, spine, and suprasellar area ATRTs have also been reported. This tumor has a strong male preponderance, and metastases at diagnosis are common.
ATRTs have a spectrum of morphologic and molecular variants, but the hallmark of this disease is the loss of the SMARCB1 (also known as INI1 , SNF5 , and BAF47 ) gene product on chromosome 22 as a result of biallelic inactivating mutations. Since the original description of monosomy 22 in ATRTs, this tumor suppressor gene has been cloned from this region and encodes a core subunit of the SWI/SNF chromatin remodeling complex. Recent publications have reported on the absence of other canonical pathway mutations and the extremely low mutation rates found in these tumors, thus demonstrating the remarkably simple genome of malignant rhabdoid tumors.
Mutations and loss of the SMARCB1 gene and protein expression may not be confined to ATRTs alone because reports on choroid plexus carcinoma, CNS PNET, and medulloblastoma have been reported, along with a recently identified entity, cribriform neuroepithelial tumor. Whether these later reports represent other diseases with SMARCB1 mutations or atypical cases of rhabdoid tumors is controversial. Other diseases such as familial schwannomatosis, multiple meningiomas, epithelioid sarcomas, and extraskeletal myxoid chondrosarcomas are known to have loss of SMARCB1. In addition, mutations in other subunits of the SWI/SNF chromatin remodeling complex (AT rich interactive domain 1A [ARID1A], polybromo 1 [PBRM1], and BRG1) have also recently been identified in neuroblastoma and ovarian, kidney, and lung cancers.
Clinical Presentation
Presenting symptoms are dependent on the age and location of the tumor. In persons with posterior fossa tumors, obstructive hydrocephalus with headaches, morning vomiting, and long tract signs is common. Because most patients are young, irritability, lethargy, or failure to thrive may be evident. In patients without closed fontanelles, rapidly enlarging head circumference is observed. Cranial nerve dysfunction is common, resulting in head tilt and diplopia. Patients with supratentorial tumors will often have obstructive hydrocephalus because of the rapid growth and concurrent cysts that obstruct CSF flow. Additional symptoms can be referred to direct compression of neural structures adjacent to the tumor and thus are dependent on location. Although uncommon spinal ATRT can occur and present with focal motor deficits. Because of the high incidence of metastatic disease, patients with symptoms related to a specific area away from the primary tumor site require thorough investigation. Although rare constitutional loss of the SMARCB1 gene (or the INI1 gene; the nomenclature is interchangeable) can occur, resulting in intracranial and metachronous extraneural disease. Patients should therefore undergo CT evaluation of the chest, abdomen, and pelvis.
Imaging and Histology
Imaging characteristics are similar to those of other neuroectodermal tumors (medulloblastoma and PNET) of the CNS, including isointense appearance on T1-weighted MRI and heterogeneity on FLAIR and T2-weighted sequences. Cystic areas are common. Tumors are usually heterogeneously enhancing with contrast administration. Because of their high cellular composition, restricted diffusion is often observed. The presence of hemorrhage and calcifications is not uncommon.
ATRT has been recognized as a distinct pathologic entity, and it is differentiated from medulloblastoma and other CNS PNETs. This distinction has been supported by findings of deletions or loss at chromosome 22q11.2, the identification of the tumor suppressor gene SMARCB1 , and the finding of germline and somatic mutations of SMARCB1 in approximately 75% of cases of CNS ATRTs, although ATRTs based on morphologic criteria with normal expression of SMARCB1 have been identified. Rhabdoid cells have the characteristic appearance of an eccentric nucleus, prominent eosinophilic nucleoli, and abundant cytoplasm, with an eosinophilic globular cytoplasmic inclusion ( Fig. 57-43 ). These cells stain with EMA and vimentin. GFAP and synaptophysin can also be positive.