High quality cancer registries are critical for any modern health care system. They provide organizations and governments with vital data for allocating public health resources in an efficient manner. They enable investigators to study cancer at both patient and population-based levels, and allow clinicians to develop optimal methods for the prevention, diagnosis, and treatment of disease. Tumor registries facilitate important comparisons between different regions, races, genders, and age groups. When a registry has been operating for many years, survival and other prognostic data become available. Trends can also be followed, which allows public health experts to project future incidence rates and predict the need for screening programs, treatment programs, and new facilities.
In the United States and elsewhere, there are 2 main types of tumor registries. Population-based registries include national, regional, or state databases that provide data on the general population. Their purpose is to capture incidence, prevalence, survival, and mortality data for all new cancer cases in a defined population.1 Hospital-based registries, on the other hand, provide data that characterize cancer care at the institutional level. This allows researchers to study specific treatment protocols and provides insight into the practice patterns at different hospitals.
One of the first organized attempts to systematically collect cancer data occurred in 1900 when the Prussian Ministry of Culture sent questionnaires to every physician in Germany. The goal of the survey was to document the total number of cancer cases, but due to a poor response rate it was regarded as a failure.1 A similar problem was also experienced in Massachusetts in the 1920s after surveys aimed at identifying cancer cases were largely disregarded by practitioners.2 In 1935, the Connecticut State Department of Health established one of the first cancer research divisions in the United States. Six years later, the Connecticut Tumor Registry was created, which subsequently became one of the first successful American tumor registries.3 To date, over 400 reports have been published with information from this state database.4
In 1942, Denmark established the first national cancer registry, the Danish Cancer Registry. In this system, Danish physicians reported cases on a volunteer basis and were given access to mortality and death certificate data.5 The World Health Organization followed Denmark’s lead in 1950 with the development of the first set of cancer registration guidelines.6 The International Union Against Cancer also created the Committee on Geographic Pathology, which emphasized the importance of recording all new cancer cases within a defined geographic population.7 With the information obtained from these initial efforts, the first volume of Cancer Incidence in Five Continents was published in 1966. The International Association of Cancer Registries (IACR) was founded later that year.8 The IACR currently serves as the membership organization for population-based registries worldwide and has set the standards for cancer registration and training for over 40 years.9
With the passage of the National Cancer Act of 1971, Congress initiated the first comprehensive, national effort to systematically collect cancer data in the United States. Citing cancer as a leading cause of death with an increasing incidence, Congress mandated that the National Cancer Institute (NCI) lead an effort to “collect, analyze and disseminate all data useful in the prevention, diagnosis and treatment of cancer, including the establishment of an international cancer research data bank.”10 In 1973, the NCI established the Surveillance, Epidemiology, and End Results (SEER) database, which has since become a leading source of information on cancer incidence and survival in the United States.11 Since its inception, the SEER program has grown substantially. Currently, it covers 26% of the US population and includes 18 different registry sites in 12 states and 6 metropolitan areas.12
To identify cancer cases, information from hospital records, nursing homes, radiotherapy units, private laboratories, and death certificates is monitored by each registry.13 Data regarding all cancer cases within a geographic area are collected by the appropriate registry and submitted to the NCI. Patient demographics, date of diagnosis, primary cancer site/type, stage at diagnosis, first course of treatment, and type of surgical treatment or radiotherapy recommended within 4 months of diagnosis are among the key pieces of information that are gathered.14
In its annual report titled the SEER Cancer Statistics Review (CSR), the NCI publishes data on the incidence, mortality, survival, and lifetime risks for specific types of cancer. For example, according to the SEER CSR, the breast cancer incidence rate from 2001 to 2005 was 68.5 per 100,000 person-years while the mortality rate was 14.1 per 100,000 person-years. Five-year survival was 88.7%, which contrasted sharply with the 60% survival rate during the early 1950s. Data regarding race are also available. For example, from 2001 to 2005, the age-adjusted death rate for white breast-cancer patients was 13.7% compared with 19.9% for African Americans.15
The NCI, the American Cancer Society, and the Centers for Disease Control and Prevention (CDC) also collaborate to publish the Annual Report to the Nation on the Status of Cancer. In the 2007 report, which included 82% of the US population, breast cancer incidence rates had decreased 3.5% per year from 2001 to 2004. This was the first consistent decrease in breast cancer incidence in nearly 2 decades.16
Independent researchers can also use SEER data. Racial and ethnic disparities in breast cancer are among the areas that have been explored. One study published in May of 2008 revealed that the age-adjusted mortality rate for African American women under 40 years of age was 2 times higher than for white women. African American women were also more likely to be diagnosed with regional or distant disease.17 Another report showed that although breast cancer incidence dropped significantly in the United States between 2001 and 2004, white women experienced a 14% decline while African American rates were essentially unchanged.18 Additional areas of investigation have included the prognostic importance of breast cancer micrometastases,19 breast cancer mortality trends according to estrogen receptor status and age,20 and outcomes following surgical resection of breast tumors in patients with metastatic disease.21
To obtain the SEER data, researchers can either query the database online using SEER*Stat software or obtain a CD or DVD. A limited-use data agreement form must be completed.22,23 There is no fee associated with accessing SEER data.
While the SEER population is comparable to the US population, there are significantly more urban and foreign-born individuals represented in SEER registries (88.2% vs 79% and 17.3% vs 11.1%, respectively).24 Individuals residing within SEER regions are also more affluent, have a higher rate of employment, and have more cancer specialists within their geographic areas.25 Additionally, the SEER data do not capture information about cancer screening, cancer detection, comorbidities, or treatment provided more than 4 months after diagnosis. To study these variables, SEER data must be linked with outside resources, such as the Medicare system.
Recognizing the limitations of the SEER data, the federal government established the SEER-Medicare database in 1991. As the primary health insurer for 97% of the US population aged 65 and older, Medicare adds a number of informative variables to the SEER dataset. Medicare files contain information regarding dates of service, diagnostic codes, procedure codes, charges for services, and reimbursement, as well as identifying data for hospitals and physicians.26 Researchers can also analyze outpatient, home health care, and hospice claims data.
SEER-Medicare data allow investigators to study cancer care along its entire spectrum. Medicare claims prior to diagnosis, such as mammography, can be used to examine cancer screening and detection methods.27,28 While SEER only reports initial treatments, Medicare claims data capture most major cancer-related procedures.29 Medicare files also contain information on the use of adjuvant chemotherapy.30,31 Disparities in diagnostic or therapeutic interventions as they relate to age, race, sex, income, location, or provider can also be measured.32,33 SEER-Medicare can also be linked with the American Medical Association Masterfile and the Unique Physician Identification Number Registry to study how provider characteristics affect the delivery of care.34
Cost is another important area that has been studied. One recent publication characterized over 300,000 patients aged 65 and older who were diagnosed with breast, lung, colorectal, or prostate cancer between 1991 and 2002. During this 11-year period, the average cost for the initial care of a breast cancer patient increased from $4,189 to $20,964. In 2002, Medicare payments for the initial care of these 4 cancers exceeded $6.7 billion.35 Survivorship,36 cancer recurrence,37,38 and end-of-life care39,40 have also been evaluated using SEER-Medicare data. Additionally, investigators have studied treatment and survival differences between Medicare beneficiaries enrolled in health maintenance organizations compared to fee-for-service plans.41,42
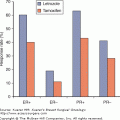
Since the SEER-Medicare database only includes information on individuals aged 65 and older, investigators must be careful when generalizing results to the rest of the population. These concerns are especially relevant when considering practice patterns, utilization rates, and outcomes for certain types of breast cancer care. For example, older patients are less likely to undergo breast reconstruction after mastectomy43 or receive axillary dissections for stage I or II breast cancers.44 Surgeons are also less likely to refer older breast cancer patients to medical oncologists.45 Additionally, while breast-conservation therapy plus irradiation is the standard of care for younger patients with early breast cancers, recent literature suggests that lumpectomy plus tamoxifen without irradiation may be sufficient for women 70 years of age or older.46
The SEER-Medicare database also lacks claims data for oral prescription drugs. This is particularly pertinent for breast cancer research since hormone replacement therapy plays a prominent role. This may change with inclusion of Part D Medicare claims data. Claims for HMO enrollees, who comprised 14% of the Medicare population in 2001, are missing in the SEER-Medicare database as well.26
Given the sensitivity of the information contained in the SEER-Medicare database, the files are not available for public use. Investigators who wish to pursue research projects must sign data-use agreements with both the Centers for Medicare and Medicaid Services (CMS) and SEER. Each research proposal must be approved prior to obtaining the data. Costs vary depending on the type of data file requested. Due to the complexity of the data files, both SEER and CMS offer assistance with data analysis.47
State cancer registries are another important source of population-based cancer data. Prior to 1992, few states had tumor registries and those that did often lacked adequate resources and legislative support.48 To address this need, the US Congress passed the Cancer Registries Amendment Act in 1992.49 This gave the CDC authority to establish and fund the National Program of Cancer Registries (NPCR). The NPCR has since provided grant money and technical assistance to state health departments for the development of statewide cancer registries. Today, the NPCR assists registries in 45 states. These data cover 96% of the US population and represent the entire US population when combined with the SEER database.50-52
The primary goals of the NPCR are to provide data for health care resource allocation, determine cancer patterns in different populations, study cancer prevention programs, and provide data to investigators. To receive funding, state registries are required to submit basic demographic information, clinical information including the date and modality of the first treatment course, and pathologic data such as tumor site and morphology. Each registry must also submit an annual statewide report to the CDC.53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree