CHAPTER 19 Tuberculosis
Introduction
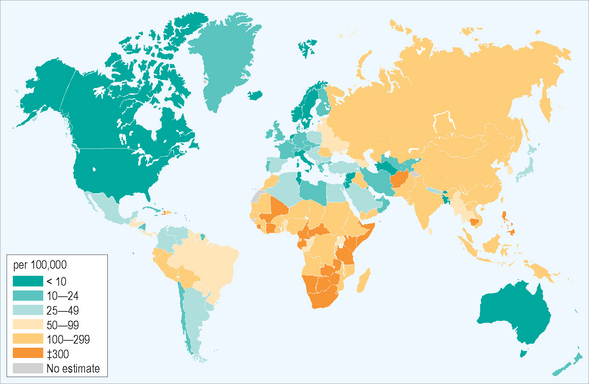
Tuberculosis (TB) remains a leading cause of morbidity and mortality in the world today. The disease is pandemic, with the highest per-capita rates now occurring in Africa, where disease incidence is increasing and a quarter of the world’s new cases develop; another half occurs in six Asian countries. In 2003, 8.8 million people around the world became ill with TB, corresponding to case rates in many countries far in excess of 100 per 100 000 of population (Fig. 19.1). More than 2 million TB-related deaths are estimated to occur annually, worldwide. Tuberculosis is the most common cause of death among HIV-infected people, especially in Africa, being responsible for nearly 250 000 deaths annually, and the disease causes more deaths among women worldwide than all causes of maternal mortality combined. Most TB deaths occur among young adults during their most productive years, providing an additional, substantial burden for developing economies.
Widespread mismanagement of infectious cases and lack of adequate prevention efforts have contributed to the increasing disease incidence and the emergence of drug-resistant strains of the organism. Multidrug resistant (MDR) disease, defined by resistance to at least isoniazid and rifampin, remains a serious problem, with almost 500 000 cases occurring each year, most in the former USSR and in China. Furthermore, within many cultures, TB is highly stigmatized, adding additional complexities to diagnosis and management.1
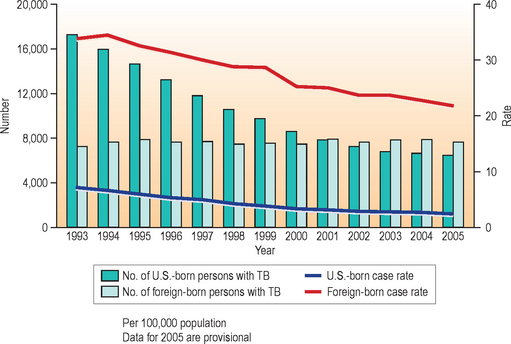
The effects of this pandemic clearly are being seen in the United States where, despite overall declines in incidence, non-US-born persons are making up an ever increasing proportion of persons with the disease. In contrast to the world situation, US cases have been decreasing in overall number since 1992. The 14,093 domestic TB cases reported in 2005 represented a case rate of 4.8 per 100 000, a 3.8% decrease from 2004. Yet that rate of decline is slowing and in 2005, 20 states reported increases in disease incidence from 2004; furthermore, non-US-born persons accounted for 54% of cases, representing a majority of US cases for the fourth consecutive year (Fig. 19.2). Among these cases, most develop TB disease within 5 years of entering the United States.2
Tuberculosis is a preventable disease for which there is good treatment. However, strategies to control TB must include four elements: (1) early diagnosis of disease, to reduce morbidity and minimize disease transmission, (2) appropriate administration of a complete course of multidrug treatment to persons with active tuberculosis for a sufficient period of time (usually 6 months or longer) to cure the disease, (3) treatment of high-risk, TB-infected persons to prevent future cases, and (4) a program of education and support for healthcare providers and for people at risk, in order to destigmatize the disease and facilitate the previous three elements.3 Many new ar-rivals have customs and beliefs that may be in conflict with these principles of TB control and may have limited resources and conflicting priorities; TB control must be made accessible and acceptable to them if the US health system is to have an impact on the disease in the United States. Furthermore, since many features of successful TB control reside outside the domain of the private healthcare sector, where many new arrivals turn for healthcare, implementation of successful TB control demands that public health resources be applied to support an infrastructure that now involves these private providers and prioritizes the effective management of TB infection and disease.
Etiology
Mycobacterium tuberculosis is transmitted in most cases by human-to-human spread of aerosolized respiratory secretions. Droplet nuclei generated by coughing or sneezing, suspended in the air, are dispersed from a source case by air currents.4 During inhalation by a naïve host, droplet nuclei from an infectious patient that contain viable mycobacteria are small enough to penetrate the alveolar ducts of the tracheobronchial tree, where they are ingested by alveolar macrophages and establish a focus of infection, which may be subclinical. Infectiousness of the source varies from patient to patient, with virulence of the organisms that cause the disease, and with susceptibility of the new host. Patients with clinical TB who have a tuberculous cavity may discharge millions of tubercle bacilli into the air with each cough, and usually are judged the most infectious.
Primary infection results when macrophages containing viable mycobacteria penetrate the alveolar wall and circulate via the blood and lymphatic systems to other regions of the body.5 During this time, usually 4–12 weeks, lymphocytes become sensitized to TB antigens, in a process that may be documented as development of tuberculin sensitivity. This is measured by conversion of the tuberculin skin test from negative to positive. Following sensitization, healing usually begins with the formation of granulomas and fibrosis at the sites to which these macrophages have migrated, as originally described by Robert Koch in 1881. However, viable bacilli remain localized within these granulomas; they alter their metabolism and enter a period of latency (dormancy) in order to survive in the inhospitable environment at these sites.6,7 Rarely, in some patients, such as children under the age of 5 and immunosuppressed HIV-infected persons, containment may not occur, and progressive, primary tuberculosis develops. This may involve primarily the lung, but usually it is a disseminated process that may involve multiple organs and may be rapidly fatal.
To healthy, non-TB-infected adults, tuberculosis generally is not highly infectious. Unlike influenza, where brief exposure may result in infection, it is believed that a relatively intense and prolonged exposure to an infectious patient, usually expressed as hours in a confined airspace where air is shared among individuals, is required for a primary infection to become established. In contrast, young children, especially those under the age of 5, or immunosuppressed persons have a greater risk of becoming infected, and then, as noted earlier, of developing disseminated primary disease, than do others. Racial and genetic predispositions to tuberculosis have never been proved; tuberculosis is a disease that does not discriminate by race.
Timely diagnosis and prompt initiation of treatment of persons with infectious TB is the best way to minimize spread to others; treatment with multiple antituberculosis medications renders most patients noninfectious within weeks. Although many cases may be treated as outpatients with appropriate case management, persons with infectious tuberculosis who may be a danger to the public’s health should be kept in respiratory isolation until they are judged to be not infectious. Medications must be taken as recommended, medical and social conditions that might impair treatment must be addressed, and patients should be educated about their disease. Nonhospitalized patients early in treatment should avoid situations where others may be exposed; they also should be instructed in how to cover their mouth/nose when coughing/sneezing in order to minimize the spread of infectious respiratory droplets. Clearly, however, prevention of development of disease is effective in reducing the need to take these measures.3
Primary tuberculosis
Clinical presentation
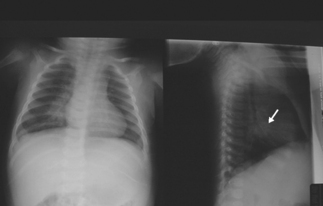
Primary tuberculosis today in the US occurs most commonly in young children; often clinically silent, it usually is discovered during the investigation of contacts of an infectious case.8 As described above, during the 4–12 weeks following inhalation of infected droplets, local tissue reactions develop in the lungs and lympho-hematogenous dissemination of tubercle bacilli occurs, along with immune sensitization. In young children, tubercle bacilli proliferate within the exudative parenchymal focus in the lung, usually located in its lower zones (middle or lower lobes, or lingula), and are carried to regional, draining lymph nodes, including those of the regional hilum (Fig. 19.3). These nodes become the site of a further inflammatory response, as sensitization develops; they become enlarged and they may compress adjacent bronchi, causing segmental lobar atelectasis, often seen as a manifestation of primary tuberculosis in children (Fig. 19.4). Central necrosis within the parenchymal focus and regional lymph nodes may occur; this process is followed by eventual healing of the lesions and, in some cases, by subsequent calcification. If the disease process continues, caseation (‘cheese-like’) necrosis in enlarged nodes may erode into bronchi and result in endobronchial spread of infected, necrotic material. The calcified lesions seen on radiographic studies at sites of healed infection and in the local nodes are the classic lesions of primary tuberculosis, termed Ghon complexes.
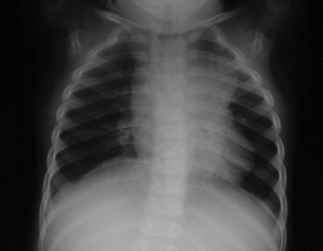
Fig. 19.4 Compression atelectasis, left upper lobe. Primary tuberculosis in a child (different patient from Figure 19.3).
Irritability or failure to thrive may be the only symptoms of primary TB disease in children; weight loss also is common. The symptoms of acute primary tuberculosis in children also may include abdominal pain as a manifestation of pulmonary or intra-abdominal disease, diarrhea, cough, and anorexia. Young children with primary TB usually are not infectious, despite symptoms. Low numbers of organisms contained in the lesions and poor generation of aerosol droplets usually are given as the reason for children being noninfectious.
Persons at risk who present to medical care with persisting (generally greater than 3–4 weeks), non-specific complaints, fatigue, or cough should be evaluated clinically for TB. The risk assessment and evaluation generally should include a thorough history, including recent travel to an endemic area, inquiry for recent contact to a known case of TB, a physical examination, with particular attention to the lungs and lymphatic system, and a chest radiograph, to rule out tuberculosis. A single, frontal view (usually posterior–anterior) chest radiograph usually is sufficient in adults.9 In children, addition of a lateral view permits visualization of hilar structures that may not be seen on the frontal projection. A positive tuberculin skin test or gamma-interferon release assay for TB (e.g. QuantiFERON-Gold®) may be helpful in supporting a diagnosis of TB, although a negative result does not exclude disease. Fifteen to 25% of patients with newly diagnosed tuberculosis fail to demonstrate a positive skin test or QuantiFERON test; therefore, these should not be used as sole screening tests for disease. Importantly, a child or adult with TB risks, such as former residence in a TB-endemic country, and a respiratory illness that fails to respond to treatment in a timely fashion should be evaluated carefully for active tuberculosis.
Latent tuberculosis
The vast majority of persons who become infected with M. tuberculosis are able to contain the organisms that become disseminated during the process of primary infection. These organisms remain in a latent state at sites of dissemination until the containment process breaks down and reactivation disease occurs – in about 10% of infected persons (see below). This period of latency offers an important opportunity to prevent reactivation TB disease from developing by treating the infection. Diagnosis is established by the tuberculin skin test or by new, blood cell gamma-Interferon release assays, such as QuantiFERON-Gold®.
The tuberculin skin test
Tuberculin, discovered by Koch around 1890, was developed into a diagnostic test for identifying tuberculous infection by von Pirquet in 1907. The tuberculin antigen used in the United States is ‘purified protein derivative’ (PPD) of M. tuberculosis, stabilized by the addition of detergent (Tween 80) to the suspension; the antigen is standardized by bioassay and is stable in its bottle if refrigerated. Intracutaneous injection of 0.1 mL of 5 TU PPD is performed using a 1 mL calibrated syringe and a 25- or 26-gauge needle. Skin, by convention usually on the flexor surface of the forearm, is cleansed with alcohol, and the needle tip is inserted just beneath the surface so that the injection creates a slight wheal (termed Mantoux technique).10,11 A tuberculin test is interpreted 48–72 hours after its application. Induration at the test site, transverse to the long axis of the arm, is palpated and is measured in millimeters; erythema is not relevant. A positive reaction, defined as a reaction greater than 5, 10, or 15 mm induration, according to risk, identifies M. tuberculosis infection (Table 19.1).
Table 19.1 Criteria for positive tuberculin skin test, by risk
| 5 mm | 10 mm | 15 mm |
|---|---|---|
| HIV-positive (known) | Recent (≤5 yr) immigration | No known risk |
| Recent close contact to infectious TB patient | Injection drug users | |
| Fibrotic changes on chest radiograph consistent with old active TB (not pleural thickening) | Residents/employees of high-risk settings (e.g. prisons/jails, long-term care facilities, nursing homes, healthcare facilities, homeless shelters, residences for HIV-infected persons) | |
| Other immunosuppression (e.g. organ transplant recipient, chronic corticosteroid therapy: equiv. of prednisone ≥15 mg/d for ≥1 mon | ||
| Persons with clinical conditions that place them at high TB risk: silicosis, diabetes mellitus, chronic renal failure, some lympho/hematologic disorders (e.g. leukemias, lymphomas), certain malignancies (e.g. carcinoma of head/neck, lung), weight loss of ≥10% ideal body weight, gastrectomy, jejuno-ileal bypass |
The BCG vaccine uses an attenuated, live strain of bovine tuberculosis; it usually is administered to children in most countries outside the US for protection against tuberculosis, although protection conferred by the vaccine likely is limited to early childhood.12 It is believed by many BCG-vaccinated people that prior BCG vaccination induces tuberculin skin test reactivity, and that a positive TB skin test in a vaccinated person is a sign of the vaccine’s effectiveness. However, the cross-reacting response to tuberculin testing following BCG, if one occurs at all, usually is small and it decreases in size rapidly following vaccination. In addition, the degree of actual protection conferred by the vaccination is highly variable, and people who receive BCG usually arrive from regions of the world where TB is endemic and a large proportion of the adult population truly is infected with M. tuberculosis. For these reasons, a positive skin test response in a high-risk person who has received BCG in the past usually is interpreted in the US as signaling true infection with M. tuberculosis, often putting the patient in conflict with his or her US provider and the US healthcare system. These issues may be settled in the future as experience is gained with new tests for TB infection (see below).
Gamma-Interferon release assays
Newer blood tests for tuberculosis infection, such as the gamma-Interferon release assays QuantiFERON Gold® (Cellestis, Ltd., Carnegie, Victoria, Australia), approved by the US Food and Drug Administration (FDA) in 2005, and the T-SPOT.TB® (Oxford Immunotech, Ltd., Abingdon, Oxon, UK), utilize antigens that are secreted by M. tuberculosis-complex organisms and a small number of other mycobacteria – but not by BCG or by common environmental mycobacteria. These tests demonstrate sensitivity similar to that of the tuberculin skin test in persons with active tuberculosis. In addition, they render greater specificity to testing for TB infection than does the skin test; this enhanced specificity has been shown in several recent studies,13–15 suggesting that current recommendations for interpretation of the tuberculin skin test in people who have received BCG may classify many skin test positive persons incorrectly as TB infected. Yet, while specificity of these tests appears greater than that of the skin test, their sensitivity for TB infection and, more importantly, their ability to identify TB-infected persons at risk for developing active TB disease is not clear, and needs to be studied. Additional studies also are needed to define the sensitivity of these tests in selected populations, such as young children and immunocompromised persons.
Blood tests for TB infection also have other limitations. These tests rely on stimulation of live, blood mononuclear leukocytes with antigen, in vitro; therefore, and importantly, blood samples must reach the testing laboratory and begin processing within hours of being drawn (currently 12 hours for QuantiFERON Gold®; 8 for T-SPOT.TB®), in order to ensure viability of the cells. In addition, these tests require special equipment and procedures, and availability may be limited in some jurisdictions. Guidelines for the use and interpretation of QuantiFERON Gold® were published recently by CDC.16 These tests have other potential benefits. They require only one visit for the blood draw and test results are not subject to the subjectivity (e.g. in interpreting the margins of a skin test induration) that has plagued the skin test. Furthermore, many people who received BCG, while familiar with the skin test and its possible confounding by BCG, do not have a similar context in which to interpret a positive laboratory test result, such as QuantiFERON. Therefore, one might speculate that they may be more likely to accept a diagnosis of TB infection if indicated by a blood test rather than a skin test.
Reactivation tuberculosis: adult-type tuberculosis disease
Pulmonary tuberculosis
The radiographic characteristics of acute tuberculous pneumonia generally are similar to pneumonia of many other etiologies, such as bacterial or fungal infections. The ‘typical’ location of pulmonary infiltrates, accompanied by risk factors for TB, immediately should raise one’s suspicion for tuberculosis (Figs. 19.5 and 19.6) (for a review of the chest radiograph in tuberculosis, see: http://www.nationaltbcenter.edu/catalogue/epub/index.cfm?tableName=RMT).17 Parenchymal infiltrates seen on a chest radiograph may be homogeneous and lobar, and may contain air-bronchograms. Tissue necrosis resulting from intense inflammation may accompany the infiltration, creating cavities within the parenchyma. A productive cough may render such patients highly infectious at this stage of their illness. Many cases heal spontaneously, resulting in focal fibrosis and lung volume loss. This in turn may result in compensatory changes within adjacent lung, such as localized emphysema, and calcification of healed foci. While treatment may speed healing, untreated disease may progress to cause respiratory failure or fatal hemoptysis.
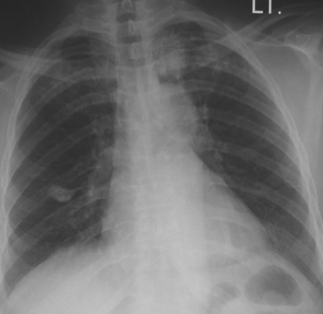
Fig. 19.5 Adult-type reactivation tuberculosis, left upper lobe. This was the source case for the patient illustrated in Figure 19.4 (grandmother). Note calcified focus left-mid lung field, likely representing site of remote, primary infection (with accompanying hilar node representing Gohn complex).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree