Trophoblastic Tumors
Jerome M. Hershman
Thyrotoxicosis occurs in patients with trophoblastic tumors, which are either hydatidiform moles or choriocarcinomas. Since the first report of thyrotoxicosis in women with a hydatidiform mole in 1955 (1), many additional cases have been reported (2,3,4,5,6,7). These reports revealed that the thyrotoxicosis disappeared rapidly after removal of the tumor, thus suggesting that the tumor produced a substance that caused the thyrotoxicosis. It is now clear that human chorionic gonadotropin (hCG) is the thyroid stimulator that causes thyrotoxicosis in patients with trophoblastic tumors.
In the United States, a hydatidiform mole occurs in about 0.5 to 2.5 per 1,000 pregnancies, and is several times more common in Asian and Latin American countries (8). Choriocarcinoma occurs in 1 in 20,000 to 1 in 40,000 pregnancies in the United States and Europe (9); about half of the cases occur in women with a previously diagnosed hydatidiform mole. Although thyrotoxicosis has been reported more often in women with a hydatidiform mole than in those with a choriocarcinoma, there have been many case reports of thyrotoxicosis in women with the latter (10,11,12,13,14), as well as in men with chorionic tumors of the testes (15,16,17,18).
The precise prevalence of thyrotoxicosis in patients with trophoblastic tumors is unknown. It was found in 5 of 20 women with trophoblastic tumors evaluated at a referral center in 1 year (19); 3 of these 5 women had a choriocarcinoma, and 2 had a hydatidiform mole. In another study, 30 of 52 women with gestational trophoblastic tumors were found to have thyrotoxicosis (2). A recent British study of 196 patients with gestational trophoblastic disease requiring chemotherapy reported that 7% had biochemical hyperthyroidism (20). In recent years, the routine use of ultrasonography during pregnancy has led to earlier diagnosis of hydatidiform moles when the tumor mass is smaller and thyrotoxicosis is less likely.
Hyperplacentosis, a rare nonneoplastic condition in which the placenta is enlarged and the serum hCG concentration is very high, may also cause thyrotoxicosis that remits promptly after delivery of the placenta (21).
Human Chorionic Gonadotropin
Human chorionic gonadotropin is composed of α- and β-subunits. The α-subunit is identical to the α-subunit of thyrotropin (TSH), luteinizing hormone, and follicle-stimulating hormone. The β-subunit of hCG has considerable structural homology with the β-subunit of TSH, but it is larger because it contains a 21-amino-acid carboxy-terminal tail.
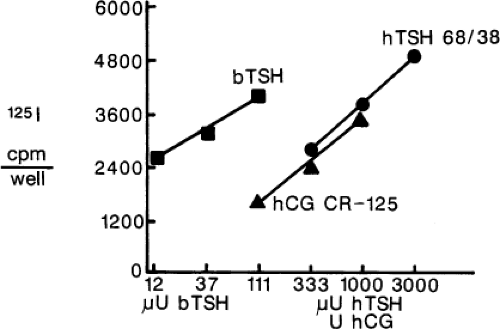
Material with thyroid-stimulating activity that has the characteristics of hCG can be extracted from hydatidiform moles and choriocarcinomas (11,22). The thyroid-stimulating activity of hCG has been demonstrated in mice, rats, chicks, and humans (22,23,24). Injection of large amounts of hCG (100,000 to 150,000 U) into normal men stimulates thyroid iodine release (23). In normal pregnant women, serum TSH concentrations decrease at 9 to 12 weeks of gestation when serum hCG concentrations are highest (see Chapter 56) (25,26,27); 18% of pregnant women have TSH <0.2 mU/L suggesting subclinical hyperthyroidism (low serum TSH and normal serum free T4 and free T3 concentrations) at that time and 2% to 3% have gestational thyrotoxicosis (27). The high serum hCG concentrations correlate with increased thyroid-stimulating activity in a mouse bioassay (26). Serum thyroid–stimulating activity measured by a thyroid cell culture assay also is increased during the first trimester in normal pregnant women, and this activity was correlated with serum hCG and free T4 concentrations (28). The thyroid-stimulating activity of purified hCG is equivalent to about 0.2 μU of bovine TSH per unit of hCG in a mouse bioassay (27) and 0.04 μU bovine TSH per unit of hCG in a rat thyroid-cell bioassay (Fig. 21.1), but is equivalent to only 0.0013 μU of human TSH per unit of hCG in a human thyroid-cell bioassay (29). Nevertheless, this thyroid-stimulating activity may be substantial in patients with trophoblastic tumors, in whom serum hCG concentrations may be as high as 2,000 U/mL. Serum hCG and T3 concentrations were correlated in women with a hydatidiform mole (5), and in five women with trophoblastic thyrotoxicosis, serum hCG concentrations were correlated with serum T4, free T4, and T3 concentrations (19). A study of 69 serum specimens with hCG exceeding 200 U/mL found that two-thirds had suppressed serum TSH, and all specimens with hCG exceeding 400 U/mL were associated with increased serum free T4 levels (30). The high serum concentrations of hCG in early pregnancy may worsen the thyrotoxicosis of Graves’ disease (31).
The thyroid-stimulating activity of hCG has been elucidated by a variety of studies. Human chorionic gonadotropin inhibits the binding of labeled TSH to its plasma membrane receptors on thyroid follicular cells (32,33), and activates adenylyl cyclase in rat thyroid cells and cells transfected with human TSH receptors (33,34). Human chorionic gonadotropin increases iodide uptake (31) by increasing the expression of the sodium/iodide transporter on thyroid cells (35).
The hCG extracted from hydatidiform moles has greatly increased thyroid-stimulating potency, as compared with hCG extracted from normal placentas (6,36). This material is enriched in the more basic forms of the molecule that contain less sialic acid than does normal hCG (6,36). Asialo-hCG purified from a patient with choriocarcinoma had potent thyroid-stimulating activity in a bioassay that used human thyroid follicles (29). Although asialo-hCG has much greater thyroid-stimulating activity in vitro than sialylated hCG, the lack of sialic acid greatly accelerates its clearance from plasma and thus reduces its physiologic action (37). Recombinant mutant hCG lacking the carboxy-terminal tail of the β-subunit stimulates the human TSH receptor about 10-fold more potently than does intact hCG (33). Presumably, the long carboxy-terminal tail and the high sialic acid content reduce the ability of hCG to activate TSH receptors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree