Fig. 29.1
This is a Venn diagram depicting the overlap patterns between TNBC, basal-like breast cancer, and BRCA-1-associated breast cancers
The luminal subtypes of breast cancer express high amounts of luminal cytokeratins and express genetic markers of luminal epithelial cells and normal breast cells [16, 17]. In contrast, “basal-like” breast cancers are so named because they tend to express cytokeratins associated with “basal” types of cancers, as they arise from the outer basal layer. Basal-like breast cancers are typically high grade and poorly differentiated when examined morphologically. Whereas the TNBC phenotype is defined by immunohistochemistry, there are no established diagnostic criteria for basal-like breast cancer on a morphological basis. In general, basal-like breast cancers are morphologically consistent with a high nuclear grade, high mitotic count, and necrosis (Fig. 29.2a), such as a grade 3, invasive ductal carcinoma, not otherwise specified. Some have the histomorphology of medullary carcinoma or metaplastic carcinoma. It has also been described that almost 82 % of basal-like breast cancers express p53, compared to only 13 % in the luminal A subgroup [14].
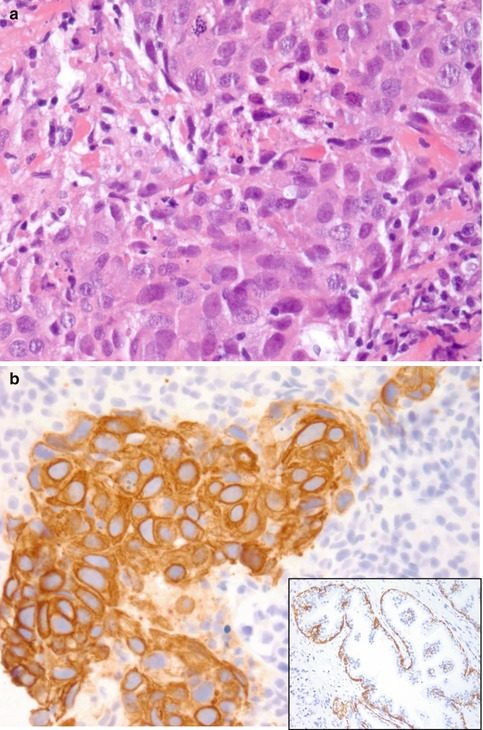

Fig. 29.2
(a) Shown is a high-grade breast cancer (invasive ductal carcinoma, not otherwise specified, grade 3), which is an example of a triple-negative breast cancer, basal-like carcinoma. Hematoxylin and eosin (H&E) staining, at ×400 magnification. (b) The above tumor showing CK5 positivity, which is typical for a basal-like cancer. Immunohistochemical CK5 staining, at ×400 magnification. Inset: CK5 stain of a normal control slide highlighting the basal cells, at ×200 magnification
There is also a subset of triple-negative breast cancer and basal-like breast cancer that is of low histological grade, such as secretory, adenoid cystic, acinic cell, or apocrine breast carcinoma. Some of the useful immunohistochemical markers for characterizing basal-like carcinomas are CK5/CK6 (Fig. 29.2b), CK14, CK8/CK18, p63, p-cadherin, vimentin, EGFR1 (or HER1), c-kit, and other growth factors such as IGFR (insulin-like growth factor receptor) [7, 17, 18].
It is also worth mentioning that not all basal-like carcinomas are HER2 negative. A study found that 23 % of basal-like tumors are HER2 positive [19]. Therefore, HER2 immunoreactivity should not be used to rule out a basal-like carcinoma. In addition, because not all triple-negative breast cancers and basal-like breast cancers are of high histological grade, the clinical management strategies outlined for high-risk triple-negative carcinomas are not always applicable. Oncologists need to be aware of this when using triple negative to define a potentially aggressive group of breast cancers. Although the majority of triple-negative breast cancers are basal-like and the majority of basal-like breast cancers are triple negative, there is a about a 25 % discordance between the two descriptive subgroups [7].
Clinical Course and Prognosis
Triple-negative breast carcinomas are known to be biologically aggressive. Although it has been suggested that they respond better to chemotherapy than other types of breast cancers, prognosis remains very poor [20]. This can be explained by two clinical factors: shortened disease-free interval in the adjuvant and neoadjuvant setting and more aggressive clinical course in the metastatic setting.
Triple-negative tumors have a very good initial response to chemotherapy, particularly with anthracycline- and taxane-based therapies. Despite this initial sensitivity, they continue to exhibit a very short disease-free survival [5, 7]. Recent studies examining the effectiveness of neoadjuvant chemotherapy in TNBC reveal that patients who have a good pathological outcome from surgery also have a good clinical response. However, those who have residual disease after completing their course of neoadjuvant chemotherapy have an overall worst prognosis compared to all other groups [21].
Carey and colleagues examined the relationship between the overall response to neoadjuvant chemotherapy and clinical outcome among three breast cancer subtypes. They used immunohistochemical profiles to classify each molecular subtype of breast cancer: group 1 was the HER2+/hormone receptor-negative (HER2 overexpressed), group 2 was hormone receptor-negative, and group 3 was HER2- (basal-like), hormone receptor-positive (luminal) subtypes. They followed a prospectively maintained dataset of patients with breast cancer treated with neoadjuvant, anthracycline-based (doxorubicin plus cyclophosphamide, AC) chemotherapy. They analyzed each subtype for clinical and pathological responses to neoadjuvant chemotherapy and examined the relationship of this response to distant disease-free survival and overall survival. After neoadjuvant AC, 75 % of patients received subsequent chemotherapy (physician dependent, but most revived a taxane) and all patients who were hormone receptor-positive received endocrine therapy [21].
Although the chemotherapy regimen administered and pretreatment stage did not differ by subtype, the clinical response to AC neoadjuvant therapy was higher among the HER2+/ER- group (70 %) and basal-like (85 %) subtypes (group 3) compared to the luminal subtypes (47 %; P < 0.0001). Pathological complete responses occurred in 36 % of HER2+/ER-, 27 % of basal-like, and 7 % of luminal subtypes (P = 0.01). Of interest, despite displaying initial chemosensitivity, patients with the basal-like and HER2+/ER- subtypes had a worse distant disease-free survival (P = 0.04) and overall survival (P = 0.02) than those with the luminal subtypes. This worse outcome among the basal-like and HER+/ER- subtypes was due to higher relapse among those patients with residual disease after completing neoadjuvant chemotherapy (P = 0.003) (Fig. 29.3) [21].
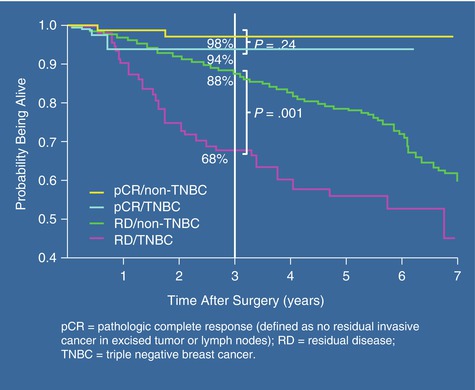

Fig. 29.3
This graph is taken from Lisa Carey’s study describing the impact of complete pathological response (pCR) on overall survival in patients receiving neoadjuvant chemotherapy. Patients with TNBC who had a complete pathological response did significantly better than those with residual disease [22]
In another study, triple-negative breast cancer was associated with an increased risk for visceral metastases (P = 0.0005), lower risk for bone recurrence (P = 0.027), and shorter post-recurrence survival (P < 0.0001) [22]. If a pathological complete response rate was achieved, patients with triple-negative breast cancer and non-triple-negative breast cancer had a similar overall survival (P = 0.24). In contrast, patients with residual disease after completing neoadjuvant chemotherapy had a worse overall survival if they had triple-negative breast cancer compared with non-triple-negative breast cancer (P < 0.0001). It is clear that patients with triple-negative breast cancer have an increased pathological complete response rate compared with non-triple-negative breast cancer patients. However, although those with pathological complete response rate have an excellent overall survival, patients with residual disease after neoadjuvant chemotherapy have significantly worse survival if they have TNBC compared with non-TNBC, particularly within the first 3 years [22].
Even for those patients with an early-stage, triple-negative breast cancer, relapse is still quite common, as high as 20 % in stage I patients over 3–5 years posttreatment. It has been noted that there is a predilection for visceral metastasis, including lung, liver, and, notably, brain metastasis. Current estimates are that approximately 15 % of patients with triple-negative breast cancer develop brain metastasis. Patients with triple-negative breast cancer have a higher risk for developing cerebral metastasis than those with other types of breast cancer. Studies show that, even in patients with cerebral metastasis, TNBC patients have a poor prognosis, as metastasis to the brain occurred earlier [23].
According to the current NCCN treatment guidelines, the appropriate management of a T1N0 (stage 1) breast cancer is based upon both tumor size and cellular characteristics. However, many oncologists will tend to treat the same group of patients who are triple negative with a more aggressive regimen of chemotherapy, in both the neoadjuvant and the adjuvant setting with the knowledge that the risk of recurrence is higher stage for stage. When the number of patients treated and the type of adjuvant chemotherapy administered were both examined, triple-negative T1N0 patients had a greater recurrence risk despite this more aggressive therapy [5]. Unfortunately, treating more aggressively with chemotherapy does not seem to help. Researchers from the Swedish Cancer Institute from Seattle, Washington, report that patients with stage 1 [T1N0] TNBC have twice the risk of recurrence, despite having received much more aggressive treatment [5]. In addition to having a very short disease-free survival, triple-negative breast tumors are aggressive in the metastatic setting, significantly contributing to the shortened overall survival [6]. Progression-free survival is estimated to be 4 months at best in patients with triple-negative breast cancer for first-line therapy, even with bevacizumab-based therapy. Final results from the bevacizumab and paclitaxel study did not show an overall benefit in overall survival [24].
Platinum-based chemotherapy is the mainstay of treatment for patients with metastatic TNBC. Multiple studies have shown the benefit of carboplatin therapy in the neoadjuvant setting as well as the adjuvant setting. In fact, there are ongoing studies, such as the Hoosier study, currently being performed that are examining the benefit of adjuvant platinum therapy combined with a PARP inhibitor in women with BRCA gene mutations and TNBC who were found to have residual disease after neoadjuvant chemotherapy (clinicalTrials gov).
Surgical Considerations in the Multidisciplinary Era
Several important steps need to be evaluated to provide a patient with TNBC optimal care. The first step in management of TNBC patients is careful consideration of the timing of the operable disease with respect to systemic therapy. This decision is of paramount importance. The lack of targeted therapy leaves clinicians and patients with very little knowledge of the effectiveness of the current systemic therapy regimens. Since the early 1990s, neoadjuvant systemic therapy for operable breast cancer has been effectively used by clinicians and basic scientists to evaluate tumor response. Pathological complete response (pCR) has been shown by multiple studies to be a useful surrogate in determining overall survival in large trials including the B-18 and B-27. The timing of the systemic therapy in TNBC as discussed by the multidisciplinary team is very important in determining responders versus nonresponders. The ability to achieve complete pathological response (pCR) varies in TNBC. However, due to high Ki-67 associated with most of these tumors, pCR can be achieved in about 22–45 % of patients, allowing for increased breast conservation rate.
Breast Conservation Therapy Versus Mastectomy in TNBC
Patients with TNBC who undergo breast conservation therapy (lumpectomy and whole breast radiation) have the same overall survival and local and regional recurrence (LRR) rates as patients who have a mastectomy [25–27]. The LRR in several studies were not significantly higher in TNBC patients than other breast cancer subtypes. The BCT was as effective as mastectomy. In a recent retrospective paper from Canada in the Journal of Clinical Oncology, TNBC patients undergoing BCT were found to have better outcome than mastectomy [28]. This was attributed to the use of radiation; however, further evaluation of this finding in prospective trials is warranted.
The issue of BRCA mutations in TNBC is very important in surgical decision making. Genetic counseling and testing in women (less than age 60) with TNBC is now the standard recommendation regardless of any family history. 75 % of BRCA1 mutation carriers diagnosed with breast cancer will have TNBC as opposed to 15–20 % (reflective of general population) of BRCA 2 carriers [29, 30]. Although local therapy for BRCA mutation carriers has shown the same LRR rates as non-mutation carriers, the risk of new primary tumors in ipsilateral and contralateral breast is four- to fivefold higher in BRCA mutation carriers [31, 32]. These patients are often counseled to consider bilateral mastectomy.
The multidisciplinary discussion of patient treatment options and screening for clinical trials are very important in our understanding of this subtype of breast cancer in hopes of finding better treatments in the future. The need for immediate surgical removal of the disease needs to be explained to the patient in the context of systemic disease processes and genetic counseling consideration to provide the patients with optimal outcome.
Hope for Targeted Therapy and Future Directions in Research
Although triple-negative breast cancer is sensitive to chemotherapy, early relapse is more likely than with other subtypes, as are visceral metastases, including the brain. Targeted agents that are currently being investigated include epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and poly (ADP-ribose) polymerase (PARP) inhibitors [24].
The anti-angiogenic agent bevacizumab (Avastin), a monoclonal antibody targeting VEGF, is active in many solid tumors including breast cancer. Miller et al. [24] demonstrated a significant improvement in progression-free survival (11.8 versus 5.9 months, HR = 0.60, P < 0.001) when bevacizumab was added to paclitaxel-based combination chemotherapy versus paclitaxel alone as a first-line treatment for metastatic disease. Examining the triple-negative breast cancer subset of patients in this study confirmed the same improvement (HR = 0.53, 95 % confidence interval = 0.40–0.70) [24, 33]. Unfortunately, long-term follow-up of this trial was not able to demonstrate a benefit in overall survival. However, there was an increase in cardiac events within the bevacizumab group, leading the FDA to deny its use for the treatment of breast cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree