Fig. 19.1
Preoperative markings for a latissimus flap
Procedure
In cases of immediate reconstruction, after the mastectomy is complete, the reconstructive surgeon must ensure that the thoracodorsal vasculature is unharmed. While dissecting within the axillary pocket, the anterior border of the LD muscle can be defined. An anterior axillary tunnel is made for the rotation of the flap into the mastectomy defect. The mastectomy flaps are then temporarily closed with staples and covered with an occlusive dressing. In unilateral reconstruction, the patient is then placed in the lateral decubitus position. If bilateral reconstruction is being performed, the patient is placed in the prone position.
If a skin paddle is being included, the skin island should be incised. The plane of dissection is taken below Scarpa’s fascia, leaving the layer of deep fat overlying the muscle as added bulk to the flap. The superior aspect of the muscle is identified at the level of the inferior border of the scapula, and the anterior margin is separated from the serratus anterior. The muscle is then raised cephalad and toward the midline. The midline attachments are then dissected. The plane in the inferior portion of the LD is less defined than the other parts of the muscle, and electrocautery is used to dissect it. The flap is then raised in the areola tissue below the muscle superiorly toward the axilla.
Once the pedicle is identified, care is taken not to injure the vessels. It is not always necessary to completely dissect the pedicle, as its arc of rotation should be adequate to rotate into the mastectomy defect even if the serratus branch is intact. The insertion of the LD at the bicipital groove of the humerus is taken down to allow for an increased arc of rotation. Great care is taken to avoid traction on the pedicle. The thoracodorsal nerve is then isolated and divided. The muscle is then freely rotated into the pocket through the previously dissected tunnel in the axilla and into the mastectomy defect (Fig. 19.2).
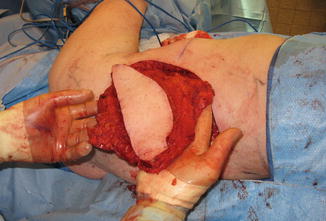

Fig. 19.2
Skin island on the isolated latissimus muscle
The donor site is closed in layers. Quilting sutures are used along with two closed suction drains. This helps to prevent postoperative seroma formation. Once the wound is closed, the patient is again placed in supine position.
The subcutaneous tunnel is made high in the axilla to help recreate the anterior axillary fold. In the case of a small breast mound, or delayed reconstruction, the flap is inset over the pectoralis major muscle. The skin paddle is then shaped, and if the entire paddle is being used, the flap can be approximated to the edges of the mastectomy flaps. Drains are placed under the flap and in the axilla. If only part of the skin paddle is being used, it is de-epithelized and inset appropriately.
In the setting of alloplastic reconstruction, the LD flap can be an alternative to the acellular dermal matrix to cover the infralateral pole of the tissue expander. The pectoralis muscle is raised and the LD is then inset at the inframammary fold. The tissue expander is then placed, with the LD flap secured to the lateral chest wall to maintain the lateral border of the breast and to keep the implant from migrating laterally (Fig. 19.3).
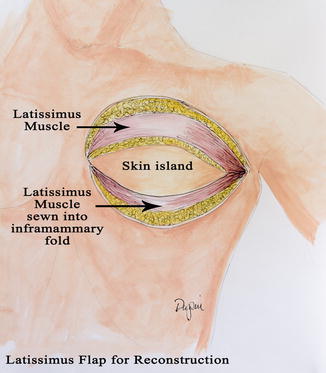

Fig. 19.3
Latissimus flap
Postoperative Course
Postoperative care usually consists of an overnight stay in the hospital. The ipsilateral arm is elevated on several pillows to prevent undue pressure on the vascular pedicle. The skin paddle is monitored for temperature and color to ensure vascular patency. The patient will go home with drains and keep a record of the output.
Complications of Latissimus Dorsi Reconstruction
Seroma formation at the LD donor site is the most common complication following LD breast reconstruction. Closing the LD donor site with quilting sutures helps close the dead space left by the dissection. Closed suction drains are imperative, with the drains kept in place for 1–2 weeks, or longer, if output is persistently elevated. Some surgeons also advocate the use of a fibrin sealant within the donor site to help seal the raw edges of the dead space. Only small retrospective studies have addressed the use of fibrin sealants, but most have noted a decrease in postoperative seroma formation as well as a shorter duration of drain usage when compared to the quilting suture groups alone [4] (Fig. 19.4).

Fig. 19.4
Preoperative and postoperative prophylactic mastectomy and implant reconstruction
Transverse Rectus Abdominis Flap
Since its introduction in the early 1980s by Hartrampf, Schleflan, and Black, the pedicle transverse rectus abdominis muscle (TRAM) has been the most commonly used form of autologous breast reconstruction. It uses the excess lower abdominal tissue that would otherwise be discarded in an abdominoplasty procedure. The use of the abdominal tissue is appealing because it is soft and moldable and therefore can be shaped to the form of a native breast. The appeal of the pedicle TRAM to surgeons is its proven reliability, predictable blood supply, and ease of harvest. However, patient selection is critical to providing good outcomes. Associated comorbidities such as obesity, diabetes, and hypertension all dramatically increase the risk of complications [4–8].
Anatomy of the Pedicle TRAM Flap
The pedicle TRAM flap is based upon the deep superior epigastric artery (a continuation of the internal mammary artery). Above the level of the umbilicus, the vessels coalesce with the deep inferior epigastric artery. There may be a significant reduction in caliber around and above the umbilicus, which impacts reliability. This anatomical variation has promoted recommendations for delay of the flap and even consideration for using both rectus muscles for transporting the flap. The deep inferior epigastric artery is a branch of the internal iliac artery and is the dominant arterial blood supply to the rectus abdominis muscle and its overlying skin. In the pedicle TRAM technique, the dominant blood supply (deep inferior epigastric artery) is ligated to allow for rotation into the mastectomy site. The valves of the deep superior epigastric veins will inhibit flow until congestion renders them incompetent [9].
The available skin is considered in four vascular zones. Numbered in order of decreasing blood supply, zone I overlays the muscle to be transposed. Zone II lies lateral to zone I and usually has a reliable blood supply stemming from zone I. Zone III is adjacent to zone I but across the midline. The viability of this zone must be explored, and portions of this zone should not be incorporated into the flap if there is any question of viability. Zone IV (the portion of the flap most remote from the vessels) is discarded in the unilateral pedicle TRAM as its perfusion is unreliable (Fig. 19.5). In bilateral procedures, each hemi-abdomen consists of zone I and zone II [10]. Basically, tissue remote to the entry of the vessels into the flap will be increasingly poorly perfused as they become further away.
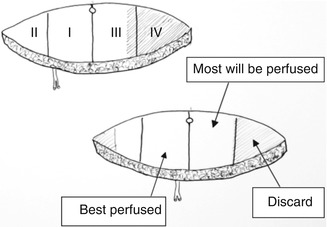

Fig. 19.5
Zones of perfusion for DIEP flap
In the case of a subcostal incision due to previous cholecystectomy, the right superior deep epigastric vessel and rectus muscle are usually compromised. Therefore, pedicle TRAM should not be raised from that side. The contralateral hemi-abdomen, however, can be considered for a pedicle TRAM transposition. Vertical midline scars do not preclude the use of the pedicle TRAM method, but tissue across the midline should not be used. A lower abdominal or Pfannenstiel incision or previous appendectomy incision do not interfere with elevation of a pedicle TRAM reconstruction [11].
Preoperative Approach and Procedure
With the patient standing, the inframammary folds are marked. The midline is also delineated. The upper abdominal marking is a transverse line 1–2 cm above the umbilicus. The lower abdominal marking is a suprapubic curvilinear incision that extends to the anterior superior iliac spine bilaterally. In the immediate setting, the mastectomy may proceed concomitantly with the TRAM dissection. The superior edge of the flap island is incised first, beveling cephalad until abdominal wall fascia is reached. The dissection is continued toward the costal margins. The medial and lateral edges of the rectus muscle are then identified. At the level of the costal margins, the anterior rectus sheath is incised on both sides of the rectus muscle down to the level of the skin island, leaving the anterior rectus sheath attached to the muscle and making the muscle dissection less difficult.
Next, the inferior border of the flap is incised down to the level of the abdominal wall fascia. The side contralateral to the rectus muscle supplying the flap is elevated first. The external oblique and rectus fascia are left intact, and large perforating vessels are ligated. The umbilicus is sharply incised to its base and separated from the flap. Attention is now directed to the ipsilateral side of the flap. Dissection proceeds until the lateral border of the rectus fascia is encountered. At this point, bipolar cautery is used to incise the rectus fascia, taking care not to injure perforating vessels. The same is done to the medial side of the rectus muscle. The medial and lateral rectus sheath incisions are extended caudally to the inferior border of the flap, where the two incisions merge. The inferior aspect of the rectus muscle is incised, and the deep inferior epigastric vascular bundle is identified and ligated. Using blunt dissection, the flap can be elevated caudally toward the costal margins (Fig. 19.6). In the area of inscriptions, the muscle can be quite thin and care must be taken not to damage the muscle and its accompanying vessels.
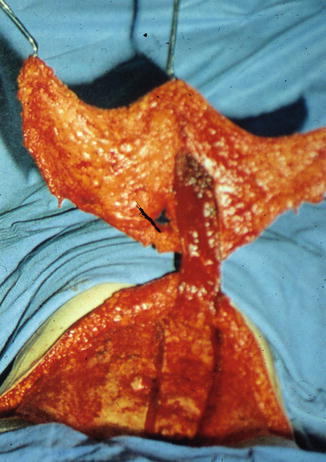

Fig. 19.6
TRAM flap
At the level of the costal margin, a tunnel is made at the medial aspect of the IMF to allow for passage of the skin island. This tunnel must be wide enough to easily pass the skin island into place without undue traction (Fig. 19.7).
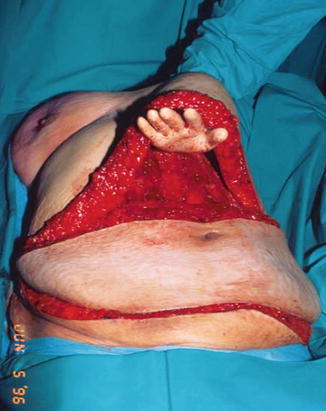

Fig. 19.7
Extensive tunnel required to transfer rotational TRAM flap
Attention is then directed to the mastectomy site, and the tunnel is completed while staying within the same plane as the dissected flap. The vascular pedicle is then identified near the level of the costal margins. Lateral attachments near the pedicle are carefully excised in order to prevent tension of the pedicle when rotated into the mastectomy site. Prior to flap rotation, the inadequately perfused part of the flap from zone IV and part of zone III will need to be excised. The flap is then rotated into the mastectomy site via the previously dissected tunnel. Care is taken to avoid any undue tension on the vascular pedicle. The flap is then temporarily stapled in the mastectomy wound.
A sound abdominal wall closure is essential in order to prevent bulging or hernia formation. The rectus fascia is closed primarily, with the superior aspect of the rectus fascia left open to prevent compression of the vascular pedicle. Once the fascia is closed, most surgeons place a mesh overlay to prevent bulge or hernia formation, a well-known complication of the pedicle TRAM. The OR table is then put into a flexed position to aid in abdominal skin closure. Scarpa’s fascia is approximated with a series of simple, interrupted absorbable sutures with placement of two suction drains. The umbilicus is repositioned in the midline and delivered through a new opening. Both the abdominal incision and the umbilicus are closed with a running subcuticular suture.
The flap is then inspected to assure adequate perfusion. It may need to be repositioned if it appears congested or compromised. Areas to be buried under mastectomy flaps are marked and de-epithelized. The flap is tacked down to the chest wall, being mindful of the borders of the breast. The skin paddle is sutured to the mastectomy flap. This allows for flap monitoring in the early postoperative period.
Bilateral Pedicled TRAM Flaps
The procedure is similar to the unilateral pedicle TRAM. The difference is in the size of the abdominal fascial defect that cannot be primarily closed. Therefore, mesh prosthesis is fashioned to the size of the fascial defect, and starting at the level of the umbilicus, the mesh is inserted. The two sutures on the opposite sides of the umbilicus set the tension of the abdominal wall repair. This is continued superiorly and inferiorly. The mesh is trimmed if necessary, and a running suture reinforces the repair of the fascial defect. The umbilicus is delivered through a hole in the mesh, and abdominal closure is completed over drains [12].
Postoperative Care
The patient is admitted to a unit capable of monitoring flaps. Temperature and color are monitored, and the surgeon is notified of any changes. The patient is encouraged to ambulate as early as postoperative day 1 and is discharged when tolerating a regular diet and when pain is controlled with oral pain medications. Patient is taught proper drain care prior to discharge.
Risk Factors in Pedicle TRAM Procedures
Smoking and obesity have been associated with deleterious outcomes following pedicle TRAM flap reconstruction. Wound healing problems, flap infections, and partial and total flap loss are greatly increased in these subsets of patients. Spear et al. reported that active smokers are at a high risk of complications with a statistically significant increase in various flap complications and infection. It was further noted that former smokers were also at increased risk of flap complications and delayed wound healing [4]. Obesity has long been considered a relative contraindication for TRAM flap reconstruction. Kroll and Netscher found that complications after pedicle TRAM flap reconstruction increased proportionally to the degree of obesity [5]. Paige et al. found obesity to be associated with an increase incidence of postoperative fat necrosis, partial flap loss, and infection. Complication rates of 31–41 % have been reported with the pedicle TRAM flap reconstruction in obese patients, with an 8–21 % incidence of partial flap loss [6]. With the introduction of the free TRAM flap, which greatly increase flap perfusion, complication rates have improved in both smokers and obese patients [6–8].
Microvascular Breast Reconstruction
Along with the development of microsurgical techniques, and in order to improve outcomes in autogenous breast reconstruction, the free TRAM (F-TRAM) flap was developed. Microsurgical transfer allows the use of the dominant deep inferior epigastric artery as the vascular pedicle to the lower abdominal flap. The more vigorous blood supply provided by the deep inferior epigastric artery decreases the incidence of fat necrosis and wound healing complications. In order to minimize donor site morbidity, the F-TRAM evolved to the muscle-sparing TRAM (MS-TRAM). The latter minimizes the amount of muscle and abdominal fascia violation, significantly reducing the incidence of hernia and bulges. The ultimate muscle-sparring TRAM is the deep inferior epigastric artery perforator flap where the perforating artery and veins are dissected through the muscle and fascia.
Anatomy of the Abdominal Wall
It is imperative to understand the anatomy of the abdominal wall in order to appreciate the fundamental difference between these procedures. The blood supply to the F-TRAM, MS-TRAM, and DIEP flap is via the deep inferior epigastric artery, a branch of the internal iliac artery. At the level of the arcuate line, the deep inferior epigastric artery often splits into a lateral and medial row after entering the rectus muscle. Occasionally there is only one vessel identified, with perforating vessels traversing the anterior rectus sheath and entering the overlying abdominal fat and skin.
Moon and Taylor described the vascular anatomy of the abdomen [10]. The primary blood supply is derived from the deep inferior epigastric artery. While there is flow across the midline to the contralateral abdominal skin and fat, it is variable. When performing unilateral reconstructions, about half of the contralateral skin and fat (zones III and IV shown above) is reliably perfused, with the remainder of the tissue discarded. In the case of bilateral breast reconstruction, the abdomen is divided in the midline so there is no concern with contralateral perfusion.
The Free Flaps Harvested from the Abdominal Donor Site
The four procedures that utilize the abdominal donor site are the free TRAM (F-TRAM), the muscle-sparing TRAM (MS-TRAM), the superficial inferior epigastric artery (SIEA), and the deep inferior epigastric artery perforator (DIEP) flap. Due to many aspects of three of the flaps being similar, only the dissection of each flap will be discussed separately.
Preoperative Evaluation and Markings
Preoperative evaluation of the abdominal wall is crucial when choosing to proceed with any abdominal tissue transfer. Close attention must be made to previous abdominal surgeries and the resulting scars. Scars in the upper abdomen increase the risk of necrosis of the abdominal donor site tissue. Scars from appendectomies and vertical midline incisions will likely jeopardize perfusion across the scar [13, 14]. Examination of the skin with an 8 MHz Doppler will give an indication of the location of the perforators. Preoperative CT angiogram is a useful adjunct that can aid in preoperative planning. Perforator size can be documented, as can their location [15, 16].
Once the dominant perforator is identified in supine position, the remaining markings are performed in the standing position. The superior edge of the flap is a transverse line typically above the umbilicus. Approximately one third of the dominant perforators are above the umbilicus [17]. It should be noted that the critical mark is the level of the cranial (superior) incision line and the dominant perforator needs to be included within the design of the flap.
The inferior edge is based on the superior marking. It is marked at a distance below the superior incision that will provide enough flap volume for the reconstruction. This may be just above the pubis or higher and extends laterally to the anterior superior iliac spine. Care should be taken to avoid making the height of the flap excessive, as this will make primary closure difficult and may result in wound healing complications. Depending upon the size of the patient, the presence of a panniculus, the size of the breast to be reconstructed, and the volume needed, the height of the flap varies from 12 to 16 cm.
In the case of immediate reconstruction, the breast skin to be excised is marked, including previous biopsy sites. The inframammary folds are marked and should be used by the oncologic surgeons as the inferior limit of dissection.
Positioning
The patient is placed in the supine position. Bilateral sequential compression devices are used and a Foley catheter is placed. In immediate reconstruction, the arm is prepped and placed out but will be tucked during the microvascular portion of the procedure. The mastectomy can occur concomitantly with raising of the flap. In delayed reconstruction, two teams are useful as while one team is dissecting the flaps, the other team dissects the recipient vessels.
Common Aspects of the Procedures
All three procedures are similar in the early dissection. The procedure begins by incising the inferior incision as marked. Frequently, there is a large (4–5 mm) vein approximately halfway between the ASIS and the midline. It is very superficial and should be carefully dissected inferiorly for a moderate distance. This vein can be used to drain the flap if there are problems with the venae comitantes of the deep inferior epigastric artery.
As the dissection proceeds deep to Scarpa’s fascia, another set of vessels are encountered. These are the superficial inferior epigastric vessels (SIEA and SIEV) that frequently emerge from the femoral artery, as a branch of the lateral femoral circumflex vessels. If this system is large (3–4 mm), it is possible to transfer the flap without dissecting the rectus muscle at all. A flap transferred on the SIEA vessels is termed an SIEA flap. Unfortunately, the vessels are usually too small to perfuse the flap and the muscular dissection must proceed.
The superior incision is made. As noted above, it should be made at least 1–2 cm above any periumbilical dominant perforator. This incision should be beveled superiorly away from the flap. A periumbilical incision is then made, and the umbilicus dissected down to the abdominal wall, leaving some soft tissue to perfuse it.
In unilateral reconstructions, the flap is dissected from lateral to medial. The dissection is rapid until the lateral rectus sheath is visualized. In order to approach the medial rectus sheath, the opposite flap will have to be raised to the midline. It is important to be sure that the flap chosen has adequate perforators on the initial side, as elevation to the midline will obviously destroy the contralateral perforators. In bilateral reconstructions, the medial rectus sheath can be approached from the midline incision as well.
If a F-TRAM is to be harvested, the fascia is incised cephalad to the skin island. Incisions are then made in the fascia on either side until the level of the lower edge of the skin island is reached. The fascia can then be opened in the midline for the caudal dissection. Next, the muscle is elevated with blunt dissection from cranial to caudal, until the deep inferior epigastric vessels are seen entering the muscle. The deep inferior epigastric vessels are then dissected to their origin. Muscular side branches are ligated using clips. The surgeon and assistant must take great care at this time to ensure no undue tension is placed on the pedicle. Once adequate pedicle length is dissected, the vessels are marked in situ with a marking pen to prevent twisting or kinking of the pedicle (Fig. 19.8). The MS-TRAM attempts to save muscle and, more importantly, fascia. The dissection is extended over the rectus fascia until the lateral row of perforators is identified. Fascia that is lateral to these perforators can be preserved. The same technique can be done on the medial side, preserving some fascia (Fig. 19.9). The muscle is split at the same level, leaving some on either side. The rest of the dissection is similar to the F-TRAM flap. If the patient is found to have dominant perforators on either row, additional fascia/muscle can be preserved by ligating the nondominant perforator row and increasing the amount of fascia and muscle spared. It should be cautioned, however, that these perforators may have long intramuscular courses and run in a transverse fashion before connecting with the deep inferior epigastric vessels. Leaving only a small cuff of muscle may injure the intramuscular vasculature (Fig. 19.9).
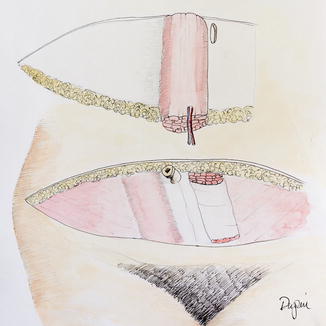
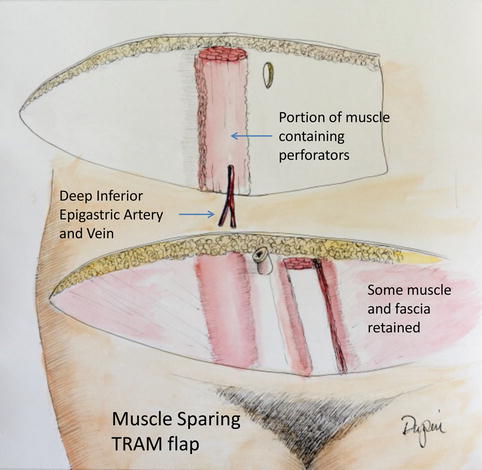

Fig. 19.8
The free TRAM flap

Fig. 19.9
The muscle-sparing TRAM flap
Deep Inferior Epigastric Artery Perforator Flap and Superficial Inferior Epigastric Artery Flap
Preoperative Planning
The success of the DIEP flap is dependent upon harvesting adequate perforators. Therefore, it is important to be certain of their size and location. Preoperatively, our patients undergo a CT angiogram of the abdominal wall. The CTA allows visualization of the dominant perforators. It demonstrates the location and also the intramuscular course of the perforating vessels. Dominant perforators are >2.5 mm in diameter at the level of the fascia. Occasionally, the ipsilateral flap is chosen if it contains dominant perforators. Flaps harvested with only small perforators, even multiple, will not be as well perfused as those harvested with dominant perforators. Occasionally, the CTA fails to identify any dominant perforators. Those patients may be more reliably converted to a MS-TRAM flap to ensure adequate circulation. The CTA also gives an estimate of the caliber of the dominant perforating vessel. Furthermore, it guides the best location of the DIEP flap designs. Saad et al. noted that nearly one third of dominant perforators were located superior and within 2 cm of the umbilicus. We move the flap design superiorly to encompass the dominant perforator [17].
In the preoperative holding area, the patient is marked with the assistance of the oncologic surgeon. Landmarks such as at the IMF and the midline are marked, and previous biopsy sites are marked for excision as well. The abdominal wall is then marked. Using a Doppler, abdominal perforating vessels are marked. The upper abdominal incision is designed to incorporate the dominant perforator. At least half of the time, it is 1–2 cm above the umbilicus. Once the level of the upper incision is decided upon, the lower incision is marked. The lower abdominal incision is marked in the suprapubic region and extends in a curved fashion to the ASIS bilaterally. Care is taken to avoid excess width of the flap. Incisional wound in very small, thin, or nulliparous patients may be closed with only a 12 cm-wide flap; those in patients with a large panniculus can be closed with a 16 cm-wide flap (Fig. 19.10).
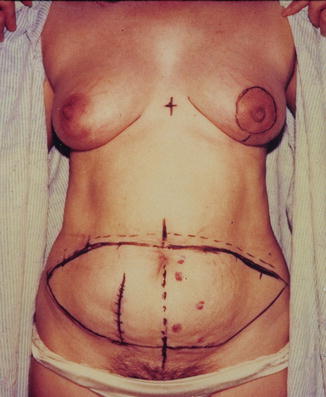

Fig. 19.10
Preoperative markings contralateral DIEP flap for left mastectomy
The patient is then taken to the operating room. Sequential compression devices and Foley catheter are placed. The patient also receives preoperative antibiotics which are re-dosed appropriately throughout the procedure.
Operative Procedure
In immediate breast reconstruction, the oncologic surgeon undertakes the mastectomy at the same time as the plastic surgeon dissects the DIEP flap. The lower abdominal incision is made first and the superficial epigastric system is explored at this time. The superficial inferior epigastric vein (SIEV) is approximately 4–5 cm lateral from the midline. If it is large in size, it should be dissected inferiorly, ligated, and preserved. Very large superficial veins may indicate that they, rather than the venae comitantes, are the primary drainage for the flap. The superficial inferior epigastric artery (SIEA) is located about 2–3 cm lateral to the SIEV but deep to Scarpa’s fascia. It is identified and examined. Occasionally the superficial system is very large (>2.5 mm in diameter). If this situation is found, it is possible to harvest the flap based on the superficial system as previously described.
A very large superficial system may also indicate that the perforators are very small. In such cases, it may be safer to harvest the flap utilizing the superficial system. This should be seen on the CTA as well. If the SIEA is large, the dissection proceeds in an inferior (caudal) direction until the SIEA is found emerging from the femoral artery. It may (48 %) join the superficial circumflex vessels before joining the femoral vessel. This is a more favorable situation as harvesting the superficial circumflex artery allows a much greater caliber vessel (2 mm) than the SIEA alone. The small size of the SIEA makes for a mismatch with the internal mammary artery (IMA), and anastomosis may be easier with a perforating branch of the IMA, rather than the IMA itself. The donor site of the SIEA flap is prone to postoperative development of a seroma, but the rectus muscle and fascia are not dissected.
If the decision is made to proceed with the DIEP flap, the superior skin incision is made and beveled superiorly to allow for more tissue inclusion in the flap and aid in abdominal wall closure. The umbilicus is dissected down to the abdominal wall. Laterally, the skin and fat is elevated from the abdomen using electrocautery. Near the lateral edge of the rectus sheath, bipolar dissection takes place and perforating vessels are preserved. If the patient is undergoing a bilateral procedure, or if a small amount of tissue needed in the reconstruction, a midline abdominal incision is made. Dissection is then taken medially to explore the medial row perforators. The periumbilical perforators can usually be visualized by dissecting caudally from the upper incision, without taking the lateral perforators.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree