Test
IHC
Fish
Positive for HER2
HER2 3+ (defined as uniform intense membrane staining of >30 % of invasive tumor cells)
HER2 amplified (FISH ratio of HER2 to CEP17 of >2.2 or average HER2 gene copy number > six signals/nucleus for those test systems without an internal control probe)
Equivocal for HER2
IHC 2+
FISH ratio of 1.8–2.2 or average HER2 gene copy number four to six signals/nucleus for test systems without an internal control probe
Negative for HER2
IHC 0–1+
FISH ratio of <1.8 or average HER2 gene copy number of < four signals/nucleus for test systems without an internal control probe
BRCA Gene Mutations
Many of the basal-like tumors share similarities with tumors from carriers of BRCA mutations. Dysfunction of the BRCA pathway interferes with DNA repair and activation of cell-cycle checkpoints and disturbs chromosomal stability. BRCA1 tumors are predominantly ER negative, a clear link observed between the basal-like phenotype and germline mutation of BRCA1 gene [65–68]. However, there are many distinct features between BRCA1-positive triple-negative tumors and the basal-like triple-negative tumors [69–75].
Tumors with a BRCA2 gene mutation have a wider range of phenotypes and are more commonly found to be ER positive. With the recent removal of patent protection for BRCA gene mutation testing in June 2013, more information on the genes and their manifestation as a prognostic and predictive factor, as well as a therapeutic target, is expected. The BRCA mutation carriers who develop ovarian cancer as a result seem to have a better survival and overall outcome after receiving chemotherapy compared to those with sporadic ovarian cancer [76, 77]. It is less clear as to the role of BRCA mutational status as a predictor of chemotherapy response in breast cancer. However, the response to PARP inhibitors for both BRCA-related ovarian and breast cancer is much higher than for sporadic tumors [78–83].
VEGF, VEGFR, PI3kinase, mTOR, and PARP
Multiple targets have been studied to match specific therapeutic interventions. Vascular endothelial growth factors (VEGFs) and vascular endothelial growth factor receptors (VEGFRs) have been evaluated in patients treated with agents targeting these ligands and receptors, with no clear association with improved outcomes clearly identified [84–86]. There has been extensive research recently that has focused on defining predictive markers for inhibitors of the phosphoinositol 3 kinase (PI3k) and mammalian target of rapamycin (mTOR) pathways, with many clinical trials examining the patients with tumors that have somatic mutations in PI3KCA [84–90].
Molecular Assessment of Breast Cancer Outcomes and Predictive Patterns by Gene Expression Profiling
Distinguished Breast Cancer Subtypes
Complimentary to the assessment of long-established markers, a large effort has been put forth by many in order to determine whether larger networks of biomarkers and pathways may provide for a better description of a specific resistance phenotype. To this end, gene expression profiling by cDNA expression microarrays has subclassified breast cancer into four distinguished molecular classes based upon similarities in gene expression characteristics [45–49].
Luminal A Subtype
Molecular profiling demonstrated that ER-positive tumors can be categorized into two distinct subtypes based upon the expression of their receptors, with tumors expressing both ER and PR considered luminal A tumors. These tumors are hormone sensitive and should be treated with hormonal therapy. Luminal A tumors are frequently less responsive to treatment with chemotherapy, which is further supported by the low pathological response rates in neoadjuvant studies [91–95]. However, given the very favorable overall survival in these tumors, chemotherapy may not be needed in patients with luminal A tumors.
Luminal B Subtype
ER + tumors with low or absent PR are considered luminal B cancers. Luminal B tumors carry a worse overall prognosis in patients. They also are found to have an aggressive phenotype, high proliferation rates, high-grade features, and resistance to hormonal therapy. The estrogen receptor signaling is often dysregulated with genomic instability, mutations, and epigenetic modification in the ER gene [47, 53, 96].
HER2-Positive Subtype
Tumors in this subtype have overexpression or amplification of HER2 and HER2-related genes on the same amplicon. In HER2-positive, early-stage breast cancer, ER is expressed in about 50 % of the patients. However, even in the setting of maintained expression of ER positivity, the predominant driver of this disease is the HER2 gene. It has been identified as a very strong prognostic and predictive molecular marker, with the specific targeting and inhibition of HER2 seen as one of the most important milestones in the treatment of breast cancer. Furthermore, the efficacy of HER2 targeting is not influenced by the expression or absence of ER. HER2 targeting with trastuzumab, or in combination with other HER2 targeting agents, should be explored in all patients with tumors that express HER2 who otherwise have no contraindications [97–99].
Nonetheless, in tumors that express both, HER2 and ER, therapy should be directed against both targets. In addition to HER2-targeting therapy, current recommendations for hormonal therapy are similar to those for ER-positive, HER2-negative tumors. While early assessment suggested that tamoxifen was less active than aromatase inhibitors in HER2-positive disease, recent studies, such as the TRANS ATAC and PHARE trials, have found similar efficacy for antiestrogens as well as aromatase inhibitors [97–100]. However, the risk of recurrence and breast cancer-related death remains high, suggesting an increase in resistance to hormonal therapy.
Basal-Like Breast Cancers
Basal-like tumors are ER-negative, PR-negative, and HER2-negative tumors, often referred to as triple-negative (TN) tumors. These tumors are genetically unstable and highly altered at the gene level. Although the majority of basal-like tumors have common morphologic features, there are no distinct features to discern prognostic or predictive patterns of therapy. Basal-like and triple-negative tumors tend to have a higher response rate to chemotherapy, but clear predictive markers to which chemotherapy is more likely to increase the disease-free and overall survival remains elusive at present.
Multigene Signature Assays
In addition to the routine assessment of ER, PR, HER2, and Ki67, several multigene signatures are currently being utilized to better predict outcomes and treatment response. The most commonly used tests are MammaPrint and OncotypeDX, which are both cleared for clinical use and have been approved by the Food and Drug Administration (FDA). The assessment and utilization of the recurrence score (RS) with Oncotype DX is endorsed by both ASCO and NCCN treating guidelines to assess the need and potential benefit of chemotherapy in early-stage breast cancer. These tests are typically used for patients with ER-positive tumors that are node-negative and are not recommended for patients with HER2-positive and triple-negative tumors.
The Oncotype DX assay was developed using real-time reverse transcriptase polymerase chain reaction (RT-PCR) for gene expression in paraffin-embedded tumors. This assay examines the expression of 21 genes (16 breast cancer genes and 5 reference genes) to estimate the 10-year distant recurrence risk of breast cancer and the potential benefit of adjuvant chemotherapy and hormonal therapy in patients with estrogen receptor-positive breast cancer [101–106]. This test has been validated from large retrospective studies of women with early-stage, estrogen receptor-positive breast cancer without lymph node involvement, with a subgroup of patients with <4 nodes involved with metastatic disease. This test was originally designed to assign a recurrence score that reflected the 10-year risk of recurrence in women with ER-positive disease with no involved lymph nodes. Patients were treated with either tamoxifen alone or tamoxifen and chemotherapy in two large NSAPBP studies (B14, n-668 and B20, n-651) [105–107]. The results of the tests are strongly influenced by the expression of the estrogen receptor and are negatively impacted by the absence of progesterone expression and a high HER2 expression. The assay may not be clinically as relevant in those patients with HER2 overexpressing and ER-positive tumors.
The assay initially grouped tumors in three groups with low risk (recurrence score 0–18), intermediate risk (recurrence score 18–30), and high risk for recurrence (recurrence score >31). Patients with a recurrence score in the low- and intermediate-risk groups were found to have limited additional benefit from adjuvant chemotherapy compared to tamoxifen alone. A special focus of the prospective studies has been to quantify the benefits of adjuvant chemotherapy in the intermediate-risk group. While initially only evaluated for women with node-negative disease, more recent studies have been expanded for women with 1–3 or >4 lymph nodes involved with metastatic disease. It also shows that they may not benefit from adding chemotherapy to hormonal therapy, as the benefits over adjuvant chemotherapy may not warrant the excess risk in toxicity [104, 108–111].
A retrospective analysis of the recurrence score in the ATAC trial evaluated recurrence scores from 1,231 evaluable patients treated with either tamoxifen or anastrozole. This trial further supported the use of the recurrence score to assess outcomes for distant recurrence in both node-positive (n = 306) and node-negative patients (n = 872), showing that the prognostic value for aromatase inhibitors is similar to those found with tamoxifen. The distribution of risk groups was similar in both groups, with the high risk representing 15 % and 17 %, respectively, of the entire group, and 59 % and 52 %, respectively, for those with a low risk based upon their recurrence score. However, the 9-year risk of distant recurrence was considerably higher in node-positive patients compared to node-negative patients, demonstrating both its prognostic and predictive value in each setting. The distant recurrence rates were 4, 12, and 25 % in low, intermediate, and high recurrence score, node-negative patients and 17, 28, and 49 % for node-positive patients. Further studies have suggested that the RS remains to be a very strong predictor for late recurrences and may be used to determine who should receive hormonal therapy beyond 5 years [112].
The impact of the Oncotype DX breast cancer assay on clinical decision making has been quite dramatic. A recent analysis of over 4,000 women revealed that the utilization of this assay altered the recommendations for adjuvant therapy in 33 % of the patients. These studies further suggested that adjuvant chemotherapy was offered in 6 % of low-risk patients, 37 % intermediate, and 83 % with high recurrence score [104, 108–110, 113–117].
Lastly, we await the results of prospective studies, such as the TailorRx and Responder trials, which will further elucidate the value of this test for clinical decision making (Fig. 22.1).
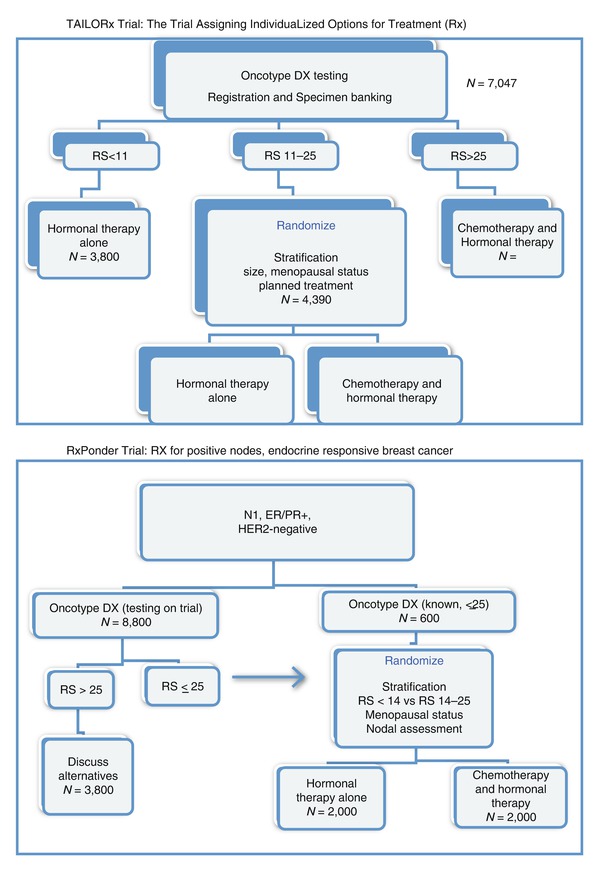

Fig. 22.1
TAILORx Trial: the Trial Assigning IndividuaLized Options for Treatment (Rx) and RxPonder Trial: RX for positive nodes, endocrine-responsive breast cancer
The MammaPrint gene assay utilizes cDNA microarrays obtained from fresh samples of breast cancer (not paraffin-embedded) to subclassify tumors into five subtypes, based upon their distinct gene expression profiles. These include luminal epithelial cell phenotypes (subtypes A and B), a basal epithelial cell type phenotype, a HER2 (+) phenotype, and a group of cancers expressing a “normal-like” gene profile. Based on earlier observations that the ER levels vary considerably between luminal or basal types [46–48], Van’t Veer and colleagues designed a 70-gene microarray platform to identify a “poor-prognosis signature” involving genes associated with the cell cycle, tumor invasion, metastasis, and angiogenesis [19, 21, 118]. An initial validation of this gene assay revealed a poor-prognosis signature as well as a good-prognosis signature. Those in the former group had a significantly worse 10-year overall survival rate [19, 21, 118, 119]. While initially developed as primarily a prognostic test, it has been further developed into a test with predictive value in adjuvant and neoadjuvant trials. Retrospective studies have suggested that the patients with a good-prognosis signature are unlikely to have a significant pathological response rate to chemotherapy [119–123].
The PAM50 gene assay test was recently approved in the United States to determine the risk of recurrence (ROR) score for patients with stage I/II, node-negative or stage II, node-positive (1–3 nodes) disease that is hormone receptor positive. A clinical challenge posed by the Oncotype DX assay is the uncertainly on whether to treat patients with an intermediate-risk recurrence score. The PAM50 assay measures the gene profile of 50 genes based on the four basic tumor subtypes (luminal A, luminal B, HER2 enriched, and basal-like) with the goal of defining a better distinction between the intermediate- and high-risk groups.
In two recent studies, tumor samples were obtained from 1,017 women with hormone receptor-positive breast cancer who received either anastrozole or tamoxifen in the TransATAC trial. The recurrence score utilizing the PAM50 (ROR) assay was then compared to the recurrence score for the Oncotype DX assay, showing that the ROR may offer a more definitive discrimination between intermediate- and high-risk groups. They identified more patients that were reclassified as high risk based upon the ROR score compared to the Oncotype DX recurrence score [124–131]. Furthermore, this test may be able to determine a group of patients that benefit from paclitaxel-based chemotherapy [126]. However, further prospective studies will be needed to validate the use of this assay in order to determine its validity and ultimate clinical use in decision making.
Conclusion
The most commonly used prognostic and predictive molecular markers for early-stage breast cancer in clinical practice are ER, PR, and HER-2, as well as Ki67. The latter remains somewhat limited at present, until further studies can assess its validity in clinical practice. Genetic assessments of BRCA mutations are more commonly used to determine the risk for developing primary tumors and to guide the use of novel agents directed against DNA damage. There are three multigene assays that are able to assist clinicians in decision making for their breast cancer patients: the Oncotype DX, MammaPrint, and, more recently, the PAM50 gene assay. Genetic assessments of BRCA mutations are more commonly used to determine the risk for developing primary tumors and to guide the use of novel agents directed against DNA damage repair.
Future Perspective
Over the last two decades, important strides have been made in our attempt to select those patients who are more likely to benefit from therapy from those that will not. Furthermore, gene assays will continue to develop that will better allow us to determine those who need therapy and define those who are less likely to develop metastatic disease. The value of many other genes as predictors of response or as therapeutic targets, as well as the assessment of proteomics and polymorphisms, is undergoing vigorous preclinical and clinical testing and will hopefully emerge as useful tools in the future. Other important predictors may arise from the evaluation of environment modifiers, next-generation sequencing, and molecular bio-imaging. These efforts will be even more important in an area of expensive and often toxic therapy.
References
1.
Beatson GT. On the treatment of inoperable cases of carcinomas of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896;2:104–7.
2.
Dao TL. Estrogen excretion in women with mammary cancer before and after adrenalectomy. Science. 1953;118:21–2.PubMed
3.
Pearson OH. Hypophysectomy in the treatment of metastatic mammary cancer. CA Cancer J Clin. 1959;9:159–62.PubMed
4.
Dodds EC, Goldberg L, et al. Oestrogenic activity of certain synthetic compounds. Nature. 1938;141:247–8.
5.
Haddow A, Watkinson JM, et al. Influence of synthetic oestrogens upon advanced malignant disease. BMJ. 1944;2:393–8.PubMedCentralPubMed
6.
Council on Drugs. Androgens and estrogens in the treatment of disseminated mammary carcinoma: retrospective study of nine hundred forty-four patients. JAMA. 1960;172:1271–83.
7.
Cole MP, Jones CT, et al. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25:270–5.PubMedCentralPubMed
8.
Ward HW. Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels. Br Med J. 1973;1:13–4.PubMedCentralPubMed
9.
Ingle JN, Ahmann DL, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304:16–21.PubMed
10.
Slamon DJ, Godolphin W, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12.PubMed
11.
Slamon DJ, Clark GM, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.PubMed
12.
Pegram MD, Lipton A, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–71.PubMed
13.
Cobleigh MA, Vogel CL, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48.PubMed
14.
Pegram MD, Konecny G, et al. The molecular and cellular biology of HER2/neu gene amplification/overexpression and the clinical development of herceptin (trastuzumab) therapy for breast cancer. Cancer Treat Res. 2000;103:57–75.PubMed
15.
Slamon DJ, Leyland-Jones B, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.PubMed
16.
Vogel CL, Cobleigh MA, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26.PubMed
17.
Pegram MD, Pienkowski T, et al. Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst. 2004;96:759–69.PubMed
18.
Slamon D, Eiermann W, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.PubMedCentralPubMed
19.
Bueno-de-Mesquita JM, van Harten WH, et al. Use of 70-gene signature to predict prognosis of patients with node-negative breast cancer: a prospective community-based feasibility study (RASTER). Lancet Oncol. 2007;8:1079–87.PubMed
20.
Modlich O, Bojar H. Can a 70-gene signature provide useful prognostic information in patients with node-negative breast cancer? Nature clinical practice. Oncology. 2007;4:216–7.PubMed
21.
Weigelt B, Hu Z, et al. Molecular portraits and 70-gene prognosis signature are preserved throughout the metastatic process of breast cancer. Cancer Res. 2005;65:9155–8.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree