Over the past century, cancer treatment has evolved into a multimodality approach, necessitating surgery, chemotherapy, radiation therapy, and/or immunotherapy. These therapies are administered alone or in different combinations and order depending on the pathology and biology of the malignancy. Most recent Surveillance, Epidemiology, and End Results (SEER) data predict that in 2016, there will be nearly 1.66 million new cases of malignant neoplasms and more than half of those diagnosed with cancer will receive radiation therapy during the course of the disease, either as neoadjuvant, primary, or adjuvant treatment.1,2
When considering head and neck cancer, radiation was primarily used as adjuvant treatment in the 1970s, but by the 1990s radiation was administered with and without chemotherapy as a primary treatment modality.3 In other cases, when given as neoadjuvant therapy, radiation is able to downstage several tumors, including soft tissue sarcoma and other solid visceral tumors. In addition, when offered with lumpectomy, radiation therapy offers a less radical surgical approach than mastectomy for certain breast cancers, producing similar outcomes. Though radiation treatment has led to significant improvements in survival and palliation in those afflicted with cancer, many of these individuals may live many years experiencing adverse acute or chronic symptomatic effects of tissue irradiation.
Though the biologic effects of radiation begin soon after treatment, the pathologic and symptomatic features may be delayed weeks to years.4 Complications of radiation therapy are said to occur in 60% to 95% of those receiving treatment.5–7 Radiation-induced skin changes have been scientifically reported for over a century.8,9 The most radiosensitive parts of the human body are the integumentary, reproductive, and gastrointestinal organ systems.10 Viability of normal tissue around the target area is oftentimes compromised, as attempts are made to ensure adequate treatment and disease control. When radiation is used for head and neck cancer, the effects include those that impair wound healing and cosmesis, and other more serious consequences as carotid artery stenosis and osteoradionecrosis. The doses used to achieve effective yet tolerable levels are based primarily on retrospective and anecdotal data, and the doses administered differ based on the tissue type, patient, and associated risk factors.4 Not surprisingly, the use of intra-arterial chemotherapy with concomitant radiation further hinders microvascular reconstruction in head and neck cancer.11
When considering just chronic wounds in general, total management costs amount to billions of health care dollars annually. Management of postradiation wounds necessitates an intricate interdisciplinary approach, involving plastic and reconstructive surgeons, nurses, dermatologists, radiation oncologists, general surgeons, and many other health care specialties.
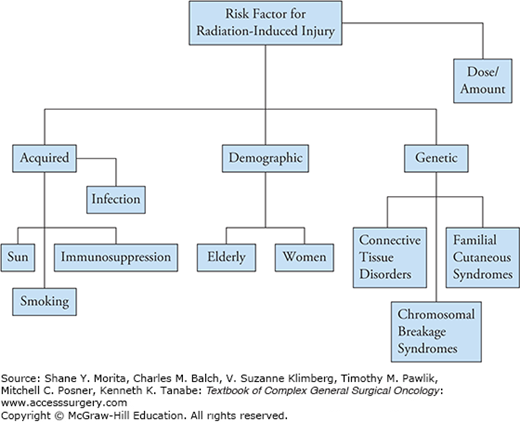
The degree of radiation injury is correlated with the total radiation dose, proportion of body and volume of tissue irradiated, and time interval of received radiation dose.10 In fact, a dose-dependent clinical pattern is seen with radiation burns, ranging from desquamation at 12 to 20 Gy to necrosis at greater than 35 Gy. Hence, fractionated doses over a longer period of time are better tolerated than local high-dose radiation. Additionally, variables related to the patient, as certain demographic, acquired, and preexisting/genetic factors, increase the risk of injury (Fig. 164-1).
Previous reports have demonstrated that female gender and older age are associated with poorer outcomes with regards to wound-healing capabilities.10,12 Additionally, with aging there is decreased cellularity, decreased collagen, and increased elastin degradation, all of which lead to increased fragility of tissues.13
Factors associated with poor wound healing include infection, obesity, smoking, chronic sun exposure, and immunosuppression, though the data supporting some of these variables are inconclusive. Immunosuppressed patients, such as transplant recipients, diabetics, and those receiving chemotherapy, are particularly at risk for nonhealing wounds. Infection is well known to impair wound healing, particularly in surgically managed irradiated wounds. In fact, classic studies have demonstrated that bacterial counts in excess of 100,000/m3 hinder skin graft survival in the surgical bed.12
Several hereditary and familial disorders make patients more susceptible to clinical sequelae of radiation injury. Polymorphisms in genes critical to cell-cycle checkpoints and regulation of apoptosis are also important. Dose alterations are required in individuals with ataxia telangiectasia (AT) and hereditary nevoid basal cell carcinoma syndrome (Gorlin’s syndrome), and therapy is unfavorable in other familial cutaneous disorders, including xeroderma pigmentosa, dysplastic nevus syndrome, and hereditary malignant melanoma.10 Minimal radiation exposure in patients with Gorlin’s syndrome can produce widespread cutaneous tissue tumors. Ataxia telangiectasia is a rare autosomal-recessive mutation in the ATM gene, and patients with ataxia telangiectasia are prone to severe dermatitis reactions after therapy. Similarly, connective tissue disorders, as lupus and scleroderma, and chromosomal breakage syndromes such as Bloom’s syndrome and Fanconi’s anemia present additional obstacles to treating an irradiated wound. Patients with Marfan’s syndrome, a defect in fibrillin protein, and Ehlers-Danlos’s syndrome, a group of disorders presenting as a defect in collagen formation, have weaker tissues and regenerative capabilities even prior to treatment, putting them at higher risk for radiation dermatitis.9 Most of the literature that associate connective tissue disorders with increased radiation-induced injury reflect anecdotal and case reports data.
The depth of penetration of radiation varies depending on type. Beta radiation can penetrate centimeters into the skin, whereas effects of gamma radiation are more extensive—through the skin and soft tissue into the body. Effects of radiation therapy can be seen at the cellular level. Typically, changes occur mainly in the nucleus at lower doses, with evident clumping of nuclear chromatin microscopically. At higher doses, the density of the nucleus increases, assuming a disfigured shape, and can show a loss of nuclear membrane.14 Derangements occur throughout the cell outside of the nucleus at these more toxic doses, altering the cytoplasm, mitochondria, and endoplasmic reticulum.14
Radiation insult leads to unorganized cellular infiltration, vascular damage, cytokine release, and subsequent fibrin leakage. The procoagulant effects, cellular clumping, and infiltration impair the dermal microvasculature network, in turn causing cellular atrophy and necrosis. The opposite is also true as dying cells lead to atrophy of their vascular supply, further inciting the cycle of injury and inflammation.
Radiation injury can also result from the generation of reactive oxygen species and specifically an imbalance of antioxidant mechanisms and redox control.10 The reactive oxygen species subsequently also lead to chromosomal damage and induction of apoptosis. In addition, injury to fibroblasts leads to decreased collagen deposition, and direct vascular injury also occurs, with endothelial cell damage and coagulation activation. Genes that play a role in this process include heme-oxygenases (HOX), glutathione peroxidases (GPX), superoxide dismutases (SOD), thioredoxins (TDX), and heat shock protein-27 (HSP27).10 Damage to the basal layer of keratinocytes also hinders the self-renewing capabilities of the skin, especially with repeated insult, as this prevents time for repair. Additionally, the production of keratin seems to be affected by injury, with a shift in expression to low molecular weight keratins (5 and 14) from high molecular weight keratins (1 and 10).6
At the molecular level, ionizing radiation incites activation signals through an unorganized release of cytokines and chemokines and stimulation of circulating immune cells and resident cells. Histologic analyses of irradiated wounds demonstrate migration of immune cells as leukocytes, macrophages, and neutrophils to injured tissue.10 Intercellular adhesion molecule I (ICAM-I), vascular cell adhesion molecule I (VCAM-1), and E-selectin are upregulated in nearby vessels.10 Vascular endothelial growth factor (VEGF), TGF-β, and Smad3 have also been implicated as mediators of chronic injury. In particular, TGF-β serves as a potent chemoattractant for various inflammatory cells and incites a fibrotic response in fibroblasts though its interaction with Smad3 receptor on these cells, causing further tissue injury. Furthermore, inflammatory and proliferative phases of the wound-healing process are disrupted, due to overexpression of proinflammatory cytokines IL-1 and IL-8, and other effects on IFN-γ and matrix metalloproteases (MMPs).6,14
Other research has demonstrated that Langerhans cells and dendritic cells are depleted from the epidermis following radiation injury.10 In mast cells, radiation leads to degranulation, and release of histamine and serotonin, as well as other molecules that significantly alter release of cytokines by dermal fibroblasts. These effects can manifest clinically as erythema and edema. Additionally, excessive production of eicosanoids (prostacyclin, prostaglandins, thromboxanes, and leukotrienes) accompanies the early and late effects of radiation toxicity.
In general, toxicity from radiation therapy can lead to cutaneous or solid-tumor malignancies and nonmalignant early or late effects.
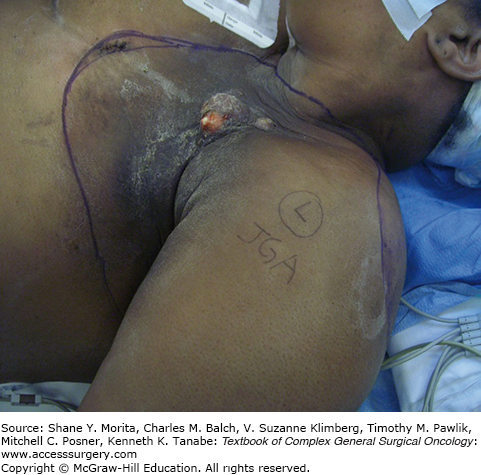
Carcinogenesis is a known unfortunate and serious consequence of radiation exposure. Sarcomas, basal cell and squamous cell skin cancers, and melanoma are just a few of the cancers that can develop after radiation exposure.9,15 Occupational exposure has even been cited with increased risk of breast cancer and leukemia.9 These malignancies generally present and are detected at a late stage, portending a poor prognosis. When considering postradiation soft tissue sarcoma alone, the occurrence is noted in a vast range of tissues, including ovarian, breast, uterine, and head and neck cancers, as well as Hodgkin’s and non-Hodgkin’s lymphoma.15 It is critical to differentiate between radiation-induced tumors and recurrence of previously irradiated cancers (Fig. 164-2).
Acute, or early, effects are those that are observed early during the course of treatment, typically within 1 month of onset.9 The wound may appear erythematous after the first week of treatment, as a result of vasodilatation and increased permeability of the vasculature. Acute radiation damage is most prominent in tissues with rapidly proliferating cells, such as in the epithelium. Examples of early effects include edema, erythema, pigment changes, and depilation (Fig. 164-3). Radiation dermatitis is classified according to a grading scale from 0 to 4, with 0 being no injury and 4 being severe injury.14 Acutely irradiated skin exhibits perivascular inflammatory infiltrate around dilated leaky vessels, sloughing of epithelial cells, and arrest of growth. Transepidermal water loss ensues, further demonstrating the loss in the integrity of the skin barrier.14 Interestingly, individuals who experience severe acute injury are not necessarily more susceptible to severe late injury.10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree